Imperial/Wet Lab/Results/Res1.6
From 2007.igem.org

Purification of GFPmut3b
Aims
To purify GFPmut3b protein to allow us to construct a calibration curve for the anaylsis of our data.
Materials and Methods
Link to the Protocol
Results
Discussion
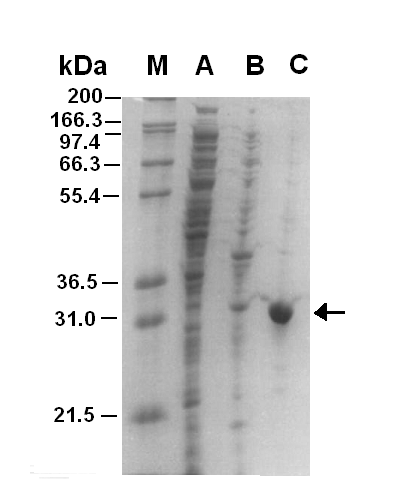
Figure 1.1 shows that the GFPmut3b was purified and eluted in fraction 17. It can be seen that there is a very large band that corresponds to the GFPmut3b. In addition, when the fractions were collected fraction 17 had an intense green color. This confirmed that the GFPmut3b purified in fraction 17 was functional. A Bradford assay was carried out on fraction 17 giving an approximate concentration of 1mg/ml for ~2ml.
Conclusion
The GFPmut3b has been successfully purified in the functional form to a concentration of 1mg/ml. This will enable us to construct a calibration curve for our in vitro chassis to correlate fluorescence with the number of molecules of GFPmut3b.