Alberta/Calender/July
From 2007.igem.org
(→July 31) |
|||
| (229 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | < | + | <b>Schedule Legend:</B> |
| - | + | JG: Jason, | |
| - | < | + | Mc: Michelle, |
| - | < | + | ED: Erin, |
| - | < | + | NG: Nick G., |
| - | + | NK: Nik K., | |
| - | + | CZ: Celine, | |
| - | + | AF: Adam, | |
| - | + | Al: Alex, | |
| - | + | JB: Jori, | |
| - | + | JP: Justin, | |
| - | + | VH: Veronica, | |
| - | + | ||
| - | + | ||
| - | + | =='''July'''== | |
| - | + | ||
| - | + | <div valign="center"> | |
| - | + | <table><tr><td><table style=" | |
| - | + | font-family: Verdana, Arial, Helvetica, sans-serif; | |
| - | + | vertical-align:middle; | |
| - | + | text-align:center; | |
| - | + | background-color:white; | |
| - | + | border-color:#FFFFFF; | |
| - | + | border-width:1px; | |
| - | + | color:black; | |
| - | + | font-size: 12px;"> | |
| - | + | <tr> | |
| - | + | ||
| - | + | ||
| - | + | <td colspan="7" style="font-weight: bold; | |
| - | + | width:170px; | |
| - | + | height:24px; | |
| - | + | color: black;">[[Alberta/Calender/July|July 2007]]</td> | |
| - | + | </tr> | |
| - | + | <tr> | |
| - | + | <td>Su</td> | |
| - | + | <td>M</td> | |
| - | + | <td>Tu</td> | |
| - | + | <td>W</td> | |
| - | + | <td>Th</td> | |
| - | + | <td>F</td> | |
| - | + | <td>Sa</td> | |
| - | + | </tr> | |
| - | + | <tr> | |
| - | + | <td>[[Alberta/Calender/July#July_1|1]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_2|2]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_3|3]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_4|4]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_5|5]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_6|6]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_7|7]]</td> | |
| - | + | </tr> | |
| - | + | <tr> | |
| - | + | <td>[[Alberta/Calender/July#July_8|8]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_9|9]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_10|10]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_11|11]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_12|12]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_13|13]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_14|14]]</td> | |
| - | + | </tr> | |
| - | + | <tr> | |
| - | + | <td>[[Alberta/Calender/July#July_15|15]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_16|16]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_17|17]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_18|18]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_19|19]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_20|20]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_21|21]]</td> | |
| - | + | </tr> | |
| - | + | <tr> | |
| - | + | <td>[[Alberta/Calender/July#July_22|22]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_23|23]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_24|24]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_25|25]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_26|26]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_27|27]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_28|28]]</td> | |
| - | + | </tr> | |
| - | + | <tr> | |
| - | + | <td>[[Alberta/Calender/July#July_29|29]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_30|30]]</td> | |
| - | + | <td>[[Alberta/Calender/July#July_31|31]]</td> | |
| - | + | <td></td> | |
| - | + | <td></td> | |
| - | + | <td></td> | |
| - | + | <td></td> | |
| - | + | ||
| - | + | ||
| - | + | </tr> | |
| - | + | </table></td><td> | |
| - | + | </td></tr></table></div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | To [[Alberta/Calender/August|August 2007]]<br> | |
| - | + | Back to [[Alberta|UofA iGEM Home]]<br> | |
| - | + | ||
| - | + | == July 1 == | |
| - | < | + | |
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 2 == | ||
| + | |||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 3 == | ||
| + | |||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 4 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Transformations of: J61003 (vector containing GFP), J23119 (constitutive promotor), E1010 (RFP), J45100 (methyl salicylate), 0034 (RBS). Transform into XL10 gold cells. Plate on Amp. | ||
| + | |||
| + | -ED, JG, ML, MC, VH, AL, CZ | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 5 == | ||
| + | |||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 6 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Transformation of E1010 did not work, it is in a Kan vector. SMRT. | ||
| + | |||
| + | Set up 4X5mL overnights of pSB1A3, J45100, B0034, J61003. | ||
| + | |||
| + | -ED | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 7 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Glycerol stocks of overnights from July 6. Stored in -80 freezer. Miniprep from overnights started July 6. Stored in "iGEM" freezer box in -20 freezer. | ||
| + | |||
| + | -JP,JB,AL | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 8 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Transform E1010 and A0500 into XL10 Gold. Plate on Kan. | ||
| + | |||
| + | Pour Kan and Amp plates. Make TBE and TAE buffer. | ||
| + | |||
| + | -ED, JP, MC, VH, CZ | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 9 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Retransform E1010 and A0050 into XL10Gold cells using new plates that were poured Sunday. | ||
| + | |||
| + | -ED, VH, JG | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 10 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Transformations of E1010 and A0050 were not successful. Tomorrow we will try with a new cell line and enriched media. | ||
| + | |||
| + | -ED, MC, AF, CZ | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 11 == | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Third attempt at transforming the Kan resistant BioBricks. Transformed E1010, A0500, and pSB2K3 into XL10 Gold, XL10 Gold with enriched media (Magnesium & Glucose), and DH5a cells. Plated 100uL and the pellet remaining after taking the 100uL aliquot on Kan plates. | ||
| + | |||
| + | Restriction digest of J61003 with Xba and Spe. Run on 0.8% agarose gel. Cut out 2290 band, will gel extract tomorrow. | ||
| + | |||
| + | <center> [[image: Alberta_e1010xlgold_july11.jpg]] | ||
| + | |||
| + | E1010 XLGOLD 10 July 11</center> | ||
| + | |||
| + | -ED, MC, NG, JG, VH, CZ | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 12 == | ||
| + | |||
| + | '''General Notes:''' | ||
| + | |||
| + | Calender up and running. | ||
| + | |||
| + | Meeting tonight at 7:00 in CSC 2-49. | ||
| + | |||
| + | Received Chlorobium tepidum, meeting with Dr. Jeff Fuller (with Capital Health) to discuss the use of their anaerobic chambers!! | ||
| + | -Justin | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Transformations with DH5a cells worked, but we will need to select for single colonies tonight. | ||
| + | |||
| + | Streak for single colonies of E1010, A0500, J61003. | ||
| + | |||
| + | Purification of gel slice of digested J61003. | ||
| + | |||
| + | |||
| + | <center> [[image: Alberta_e1010DH5a_july12.jpg]] | ||
| + | |||
| + | E1010 DH5a July 12</center> | ||
| + | |||
| + | -ED, JG, AL, JB, MC, CZ | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 13 == | ||
| + | |||
| + | '''General Notes:''' | ||
| + | |||
| + | Everyone who is coming tonight please be present 7:00pm sharp | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Started 4X5mL overnights of E1010 and I0050 at 8:30 am. Should be ready for miniprep tonight. | ||
| + | |||
| + | -ED, JB | ||
| + | |||
| + | No growth in over nights - left these shaking in 37 incubator because they may be "slow growers"<br> | ||
| + | Started new overnights of E1010, I0500, and psb2k3 (all DH5alpha) at 2200hrs incubating in the incubator by the autoclave - plates were also streaked (&labeled corresponding to the tubes)<br> | ||
| + | Also made overnights for massive colonies seen on 48hr growth of E1010 in XL<br> | ||
| + | Made some Kan plates<br> | ||
| + | |||
| + | -MC, JP, JG, CZ | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 14 == | ||
| + | <b>Schedule:</b> | ||
| + | |||
| + | AL,JP,NK | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | If you are scheduled during the day please be in the lab at 1230hrs (PM) | ||
| + | Don't forget that the Bio-Sci doors lock at 1900hrs so if you are trying to get in after 1900hrs call 492-2911 | ||
| + | |||
| + | Things to be continued today (CZ)<br> | ||
| + | 1. Put LB plates in 4 degree fridge <br> | ||
| + | 2. Plate 20 microlitre of DH5 alpha on a kan plate as a control<br> | ||
| + | 3. Xba1/EcoRI double digest <br> | ||
| + | NOTE: Sequential digestion is needed. Start with 1 microlitre of Xba1 and 2 microlitre of 10x Tango and incubate for an hour. Then add 1 microlitre of EcoRI and 2.5 microlitre of 10X Tango and incubate for an hour. <br> | ||
| + | 4. Miniprep on the colonies picked on July 13 at 10pm. They are in the shaker in the back room. Note the colonies picked were also streaked on kan plates in the 37 degree incubator. Erin's colonies are in the shaker in the front room.<br> | ||
| + | |||
| + | If you need anything else call me(Celine). Number on the blackboard. Thank you.� | ||
| + | |||
| + | <b/>Lab Notes:</b> | ||
| + | JP, AL, NK, JB(?) <br> | ||
| + | |||
| + | 1. Completed Mini on E10050 (4), I0500 (2), PSB2K3 (2) samples (the two labeled Erin, are those from July 13 8:30am ON's) In DNA box -20 <br> | ||
| + | 2. Completed sequential double restriction on J61003 promoter region. Used Xba1 and EcoR1 (see protocol). sample in DNA box -20 <br> | ||
| + | 3. Placed Kan plates at +4 <br> | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 15 == | ||
| + | <b>Schedule:</b> | ||
| + | |||
| + | Jp,JB,AL | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | Options: <br> | ||
| + | |||
| + | 1. complete double restriction on newly mini'd plasmids (arabinose promoter, RFP)<br> | ||
| + | 2. gel purify J61003 restriction products (promoter restriction sample) <br> | ||
| + | 3. fundraising letters! <br> | ||
| + | |||
| + | <b>Lab Notes:</b> | ||
| + | |||
| + | 1. ran a gel containing the digested products of the J61003 (promoter digest), snapped photo and cut out bands for extraction/purification tomorrow<br> | ||
| + | 2. glycerol stocks of Chlorobium tepidum<br> | ||
| + | 3. finished grant application forms<br> | ||
| + | - JP, AL, NG, MC | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 16 == | ||
| + | <b>Schedule:</b> | ||
| + | |||
| + | CZ,VH,AF | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | 1. Because we didn't get into lab till late (because of locked doors), did not complete restriction on newly mini'd plasmids. Can be completed today (isolate arabinose promoter and RFP) <br> | ||
| + | 2. All of the restriction product (for J61003 XbaI/EcoR1) were ran and cut - no sample remaining <br> | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Completed gel extraction of E,X digested J61003. (in box in -20) | ||
| + | |||
| + | Restriction digests of E1010 (X,S) and I0500 (E,x), ran on 0.8% gel. E1010 expected a band at 680bp, I0500 expected a band at 1200 bp. Got two positive lanes for E1010: XL1 and XL2 minipreps. Cut out bands and put in -20. No positive for I0500. | ||
| + | |||
| + | -VH, ED, CZ | ||
| + | |||
| + | |||
| + | '''Quotes of the Day:''' | ||
| + | |||
| + | "If I had a remote, I'd change the cd." | ||
| + | |||
| + | Anonymous, in response to the skipping music on the cd player, but much to lazy to get up and change it | ||
| + | |||
| + | "I would never drive this tired. But I WILL operate chemicals." | ||
| + | |||
| + | Anonymous | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 17 == | ||
| + | <b>Schedule:</b> | ||
| + | |||
| + | ED,VH,AF | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Gel extracted E1010 from yesterday's slices. | ||
| + | |||
| + | Mini-prep on A0500 from a culture labelled with red marker. If anyone knows what cell line is in this culture, please post. <br> | ||
| + | <b> It is I0500 and it is DH5alpha unless otherwise labelled . .. only the E1010 had both DH5alpha and XL10gold overnights -MC </b> <br> | ||
| + | |||
| + | Let's not do anything more with it then, because the DH5as aren't working due to them already having their own Kan resistance. - ED | ||
| + | |||
| + | Ligation of E1010 (RFP) into J61003. Used 4 different ligation conditions. These are sitting on the instructor's bench at the front of the room. These need to be transformed into XL10 Gold and plated on Amp tomorrow. Try transforming 1uL and 10uL. | ||
| + | |||
| + | -ED,JG,VH,AF | ||
| + | |||
| + | '''Quote of the Day''' | ||
| + | |||
| + | "Cancer is an urban myth." | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 18 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | CZ,MC,NG | ||
| + | |||
| + | <b>Lab Notes:</b> | ||
| + | |||
| + | Transformed Ligations 1,2,3,4 into XL10 Gold Competent cells <br> | ||
| + | Plated 1ul,10 ul, and 200ul on AMP <br> | ||
| + | Incubated @ 37celsius overnight<br> | ||
| + | |||
| + | -MC, NG, CZ | ||
| + | |||
| + | <b>Quote(s) of the Day:</b> | ||
| + | |||
| + | "Asuuuuuuuuuuuum!!" <br> | ||
| + | In response to the awesome; "You're wayyyy to exciting for me right now" | ||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 19 == | ||
| + | |||
| + | Meeting at 7:00, CAB 373. | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | ED,MC,JG | ||
| + | |||
| + | <b>General Notes</b> | ||
| + | |||
| + | New Schedule is up<br> | ||
| + | check out the jar o' love @ the front :) <br> | ||
| + | |||
| + | <b>Lab notes</b> | ||
| + | |||
| + | Started 8X5mL overnights to test colonies of the RFP ligation. Hopefully JB will be available to do minipreps tomorrow. Miniprep protocol is tucked in Master Book. | ||
| + | |||
| + | -ED,JG,MC | ||
| + | |||
| + | <b>Quote of the Day </b> | ||
| + | |||
| + | "There is a pee throw up smell it the washroom" | ||
| + | |||
| + | Reply | ||
| + | |||
| + | "Someone must be pregnant" | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 20 == | ||
| + | <b>Schedule:</b> | ||
| + | |||
| + | JG,ED,MC <br> | ||
| + | |||
| + | <b>General Notes:</b><br> | ||
| + | |||
| + | There is now a Jar O' Love in the lab; Please do not drink chemicals from said jar o'love. Check it out - it's at the front on the instructors bench. It's to give shout outs for people who are rocking out and 'taking one for the team'. <br> | ||
| + | |||
| + | <b>Lab Notes:</b> | ||
| + | |||
| + | Miniprep Lig3 (8 samples) overnights - JB<br> | ||
| + | Made 1L LB broth (16 bottles) at the back to be put away in the sterile cupboards tomorrow <br> | ||
| + | Ran Gel of JB's Minipreps after digestion with Xba and Spe. All of the colonies were positive (band at 1.2 kB).<br> | ||
| + | Did some dishes - we should learn how to use the dishwasher<br> | ||
| + | |||
| + | - MC, ED, JG <br> | ||
| + | |||
| + | <b>Quote(s) of the day:</b><br> | ||
| + | |||
| + | "Always use protection when using the Jar O' Love" | ||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 21 == | ||
| + | <b>Schedule:</b> | ||
| + | |||
| + | JP,NK,AL | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | |||
| + | Since it doesn't look like Nik will be showing up, maybe just call Alex and see if it works for him. | ||
| + | |||
| + | All of the ligations of RFP into J61003 turned out. They are labelled JB July 19 1-8 in the DNA box. Transform 1 of them into XL10 Gold and plate on Amp with Tetracycline plates. You will have to add the Tet to the plates beforehand. I don't know what concentraion, but James suggested 1 ug/mL. Try plating different amounts. | ||
| + | |||
| + | The reason that I am making these suggestions is that I read that XL10Golds are Tet resistant, so we can maybe induce the expression of RFP with the Tet because it is under the Tet promotor and then we can see the RFP. | ||
| + | |||
| + | As always, call me if you need anything. | ||
| + | |||
| + | -ED | ||
| + | |||
| + | <b>Lab Notes:</b> | ||
| + | |||
| + | 1. got into building <br> | ||
| + | 2. waited for peers - no shows <br> | ||
| + | 3. tried to break into room by climbing through roof (cement goes all the way up) <br> | ||
| + | 4. went home... will complete work tomorrow - sorry i'll get swipe access asap... <br> | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 22 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | JP,AL,NG | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | How does 7:00pm sound? | ||
| + | |||
| + | <b>Laboratory Notes:</b> | ||
| + | |||
| + | Transformed XL10 gold cells with J61003 (with RFP ligated in). Split cell aliquot into two 50microL aliquots and added 1microL DNA from ligation sample 1 and from ligation sample 4. Covered AMP plates with 50microL 1microg/ml tet, spread, let sit. Plated (1) 50microL sample and (1) 5microL + 45microL LB onto Tet/Amp plates for #'s 1&4. They are in 37C incubator. The remaining transformed cells are in +4C labeled July 22. <br> | ||
| + | |||
| + | <b>Serious Quote of the Month</b> | ||
| + | |||
| + | <b>Faust</b><br> | ||
| + | Then shall I see, with vision clear, <br> | ||
| + | How secret elements cohere, <br> | ||
| + | And what the universe engirds, <br> | ||
| + | And give up huckstering with words <br> | ||
| + | |||
| + | -Johann Wolfgang von Goethe <br> | ||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 23 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | VH,CZ,JG | ||
| + | |||
| + | <b>Laboratory Notes:</b><br> | ||
| + | 1. Add more Tet on ligation plates from July 22. Hopefully red colonies will appear!<br> | ||
| + | 2. Transformation of XL10Gold with<br> | ||
| + | - Butanol CoA dehydrogenase (1 in 1000 dilution)<br> | ||
| + | - Butenoyl CoA dehydrogenase (1 in 1000 dilution)<br> | ||
| + | - J23018 (RFP positive control)<br> | ||
| + | |||
| + | 20 and 100 microlitres of transformed culture plated on Amp plate and incubate at 37 degrees<br> | ||
| + | |||
| + | The leftover mixture is stored in 4 degree fridge in case of no growth.<br> | ||
| + | 3. Repeate overnight ligation from July 22. The exact amounts of insert, vector etc. are on the blackboard.<br> | ||
| + | |||
| + | For tomorrow:<br> | ||
| + | 1. Pick colonies for transformed plates.<br> | ||
| + | 2. Plate ligation mixtures with appropriate amount of Tet.<br> | ||
| + | 3. Observe red colonies from the July 22 ligation plates if the cells are still alive.<br> | ||
| + | |||
| + | Memorable Quote:<br> | ||
| + | Don't force it. Let it come out naturally.<br> | ||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 24 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | AF,ED,NG | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Transformed I0500 (arabinose promotor) into HB101 competent cells. Plated 2, 20, 200 uL on Kan. | ||
| + | |||
| + | Transformed ligations from yesterday into XL10 gold. Plated 100 uL on Amp. | ||
| + | |||
| + | RFP control from yesterday worked, but sadly our ligations are not expressing any RFP. It was still exciting to see red control cells. It seems as if the Tet promotor is induced at a low level without any Tet. | ||
| + | |||
| + | Started 4X5ml overnights of enoyl coA hydratase and butyryl coa DH. There seems to be some confusion with the naming of these proteins. These are the names that I have used in the BioBricks. We will be able to tell if they are correctly labelled when we do the digests. Enoyl coa hydratase is about 840 bp and butyryl coa DH is about 1200 bp. | ||
| + | |||
| + | Note on agarose gels: Use only the wide combs (8 lanes) because we are having to many problems with the narrow lanes. This may mean that you will need to pour multiple gels (oh my). Also, try and run two ladders per gel until we get the ladder problem sorted out. Also, make sure that you zoom in on the gel when taking a picture. | ||
| + | |||
| + | Note on Tet: should be stored 12.5 mg/mL in 50% ethanol, 50% water solution. This is a 1000X solution. Keep this in mind when using Tet again. | ||
| + | |||
| + | -ED, AF, James | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 25 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | Jp,MC,VH | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | Use of wide combs- 5microL of sample works well with 3.5microL of ladder. Make sure you stir up the ladder before use!<br> | ||
| + | |||
| + | '''Lab Notes:''' | ||
| + | |||
| + | Completed minipreps of enoylcoa hydratase and butyryl coa dh. They are in the new box with the green tape. I think that there are two types of digests we should be doing: one to make sure we have the right stuff (digest with EcoR1 and Pst1) and one to clone in the RBS. To clone in the RBS, cut B0034 with Eco and Spe and cut the enoyl coa hydratase and butyryl coa dh with Eco and Xba. | ||
| + | |||
| + | Check to see if any colonies have grown on I0500 plates in incubator. There weren't any visible on Adam's transformations this morning. James also transformed some this morning, so they may be hard to see. If there are colonies, start overnights and see if Jori can do minipreps tomorrow. If Jori can't make it, get ahold of me and I can. AND if there are no colonies, just post here and I'll go check the plates in the morning. | ||
| + | |||
| + | I also forgot to start glycerol stocks of our enoly coa hydratase and butyryl coa dh. If you guys could restart some overnights from the plates in the fridge so I can do it tomorrow that would be great. | ||
| + | |||
| + | I had to rush to work this morning and didn't get any of this written in the lab book. So you guys can make fun of me or whatever. I guess I belong in the Science Hall of Shame. | ||
| + | |||
| + | -ED <br> | ||
| + | |||
| + | Enny = Enoly CoA Hydratase<br> | ||
| + | Benny = Butytyl CoA DH | ||
| + | |||
| + | Checked plates<br> | ||
| + | - Adam - no growth: parafilmed and put in 4 fridge<br> | ||
| + | - James'? J23018: red colonies parafilmed and put in 4 fridge (was this the control?)<br> | ||
| + | |||
| + | Overnights of Benny & Enny<br> | ||
| + | - 3x 5mlLB each <br> | ||
| + | |||
| + | Restrictions<br> | ||
| + | - B0034 with SPE & ECO<br> | ||
| + | - Enny with Xba & ECO<br> | ||
| + | -Benny with Xba & ECO<br> | ||
| + | These are stored in the -20 freezer<br> | ||
| + | |||
| + | - MC, NK, VH, NG, JP<br> | ||
| + | |||
| + | <b>For Tomorrow:</b><br> | ||
| + | - Glycerol stocks of Enny & Benny (these colonies are likely different from the transformations as it was not marked out which colonies were picked for transformations)<br> | ||
| + | - Gels for restrictions of B0034, Enny & Benny | ||
| + | - cut out the bands; gel extractions? (if there is time)<br> | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 26 == | ||
| + | |||
| + | Meeting at 7:00, Room CAB 373. | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | AL,VH,ED | ||
| + | |||
| + | <b>Lab Book</b> | ||
| + | |||
| + | Ran gel of "Enny", "Benny" and Boo IV against 1 kb ladder. | ||
| + | |||
| + | Cleared up glasswares in the autoclave. | ||
| + | |||
| + | Parafilm all unparafilmed plates. | ||
| + | |||
| + | Checked out red fluorescence of J23018. | ||
| + | |||
| + | Attempted working on fundraising letters (?) | ||
| + | |||
| + | -ED, VH, AL, JG, MC, AF, Doug | ||
| + | |||
| + | <b>Favourite "F" word of the day:</b> | ||
| + | |||
| + | fulcrum | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 27 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | JP,MC,NK<br> | ||
| + | The theme is Foul music Friday - bring any bad music you have (or embarassed that you have in your collection) | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | 1. This is whats on the agenda for this evening: | ||
| + | [[Image: Alberta_promoterprob.jpg]] | ||
| + | |||
| + | <b>Lab book:</b> | ||
| + | |||
| + | Digest Enny(1,4), Benny (1,2) B0034(2,4) with PST, XBA, SPE<br> | ||
| + | Enny with PST and XBA<br> | ||
| + | Benny with PST and XBA<br> | ||
| + | B0034 with PST and SPE<br> | ||
| + | O/N of Benny and Enny glycerol stock (3 each)<br> | ||
| + | |||
| + | '''''Note the 4 degree fridge at the back is out of commision. All iGEM materials have been moved to the room where we take pictures of gels. The door labelled IGEM.''''' Nik is here on saturday and Celine can show on sunday.<br> | ||
| + | |||
| + | - JG, NK, MC, JP, and James (cos he loves us) | ||
| + | |||
| + | |||
| + | <b>Jame's Fun Tip of the Day:</b> | ||
| + | If you want to run a gel for a shorter period of time ad the EtBR to the gel rather than the buffer. You can add ~4ul to the gel rather than 10ul to the buffer. | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 28 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | CZ,JB,NK | ||
| + | |||
| + | <b>Meeting @ 1300hrs - MC on behalf of CZ</b> | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | To Do:<br> | ||
| + | - Glycerol stocks of Enny & Benny from the O/N<br> | ||
| + | - Gels of Enny, Benny & B0034 (put the EtBr in the gel)<br> | ||
| + | - Ligations of Enny+Benny into B0034 with T4 ligase; leave O/N in incubator at the back room (near the phone) set at 13degrees celsius | ||
| + | |||
| + | <b>Lab Book</b> | ||
| + | |||
| + | Finished making glycerol stocks of Enny and Benny from the O/N. Followed Erin's protocols except the Flash Freeze step.<br> | ||
| + | |||
| + | Ran agarose gel of Benny, Enny, b0034 digest.<br> | ||
| + | No DnA ladder (NEVER DO IT AGAIN). <br> | ||
| + | Ran at 90V for 20 Min, run longer for next time.<br> | ||
| + | |||
| + | Cut Benny band (1200BP) and B0034, need to redigest Enny tomorrow<br> | ||
| + | Gel extration using QIAGEN kit<br> | ||
| + | |||
| + | Ligation , control only with ligase, <br> | ||
| + | Stored at 13 degrees celsius overnight <br> | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 29 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | CZ,AF,NG<br> | ||
| + | |||
| + | <b>As agreed on Thursday meeting at noon or 12</b> | ||
| + | |||
| + | <b>General Notes:</b> | ||
| + | |||
| + | 1. Transformation of ligation (Benny and B0034( into XL10 Gold <br> on Amp plates; incubate at 37 degrees. Hopefully we have colonies to pick tomorrow.<br> | ||
| + | 2. Double digest of Enny (Pst/Xba) and B0034 (Pst/Spe) repeated; Enny band (800bp) and B0034 (2.7kb) bands cut out and purified; overnight ligation mixture at 13 degree incubator at the back of the room <br> | ||
| + | |||
| + | |||
| + | For tomorrow:<br> | ||
| + | 1. Pick colonies of Benny and B0034<br> | ||
| + | 2. Transformation of Enny and B0034<br> | ||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 30 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | VH,JP,JG, | ||
| + | |||
| + | <b>Lab SHTUFF</b> | ||
| + | |||
| + | 1- started overnights on 10 different colonies benny colonies(see lab book for details)<br> | ||
| + | 2- transformed new enny's and one boo34 control (plates at 37 degrees)<br> | ||
| + | 3- made new amp <br> | ||
| + | 4- sent out sponsorship letters! <br> | ||
| + | |||
| + | <b>Sports quote of the Day </b> | ||
| + | |||
| + | You train to play good, you hope to play good, I think we played like bad@zz'z <br> | ||
| + | |||
| + | |||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | == July 31 == | ||
| + | |||
| + | <b>Schedule:</b> | ||
| + | |||
| + | AF,JP,MC | ||
| + | |||
| + | <b>Laborious Notes:</b> <br> | ||
| + | 1:Restriction of Boo34 with SpeI and PstI (Now we have tons of stock restricted plasmid just incase and we are SURE that these have been restricted to the maximus!<br> | ||
| + | 2:mini preps on all of the 'Benny in boo34' overnights along with glycerol stocks of each <br> | ||
| + | 3:poured a fancy gel for tomorrows usage (EtBr in gel) <br> | ||
| + | 4:started overnites on 'Enny in Boo34' (plates in 4 degrees) <br> | ||
| + | 5:transformed TWO NEW GENES BuOH DH ("Buddy") and BBbhBu_CoA DH ("Betty") into golden bacteria (at 37 degrees) <br> | ||
| + | 5a: Buddy & Betty are in 1.5ml tubes in the 4degree fridge incase the plates do not show growth. | ||
| + | |||
| + | <b>Rule of the day</b> | ||
| + | |||
| + | No Touching. | ||
| + | |||
| + | <b>Fact of the day</b> | ||
| + | |||
| + | Vortexing after lysis is not like getting a bad haircut. Hair grows back. | ||
| + | |||
| + | [[Alberta/Calender/July#July|to the top]] | ||
| + | |||
| + | |||
[[Alberta|UofA iGEM Home]]<br> | [[Alberta|UofA iGEM Home]]<br> | ||
[[Alberta/Calender/June|To June 2007]]<br> | [[Alberta/Calender/June|To June 2007]]<br> | ||
[[Alberta/Calender/August|To August 2007]] | [[Alberta/Calender/August|To August 2007]] | ||
Latest revision as of 03:22, 1 August 2007
Schedule Legend: JG: Jason, Mc: Michelle, ED: Erin, NG: Nick G., NK: Nik K., CZ: Celine, AF: Adam, Al: Alex, JB: Jori, JP: Justin, VH: Veronica,
July
| ||||||||||||||||||||||||||||||||||||||||||||||||||
To August 2007
Back to UofA iGEM Home
July 1
July 2
July 3
July 4
Lab Notes:
Transformations of: J61003 (vector containing GFP), J23119 (constitutive promotor), E1010 (RFP), J45100 (methyl salicylate), 0034 (RBS). Transform into XL10 gold cells. Plate on Amp.
-ED, JG, ML, MC, VH, AL, CZ
July 5
July 6
Lab Notes:
Transformation of E1010 did not work, it is in a Kan vector. SMRT.
Set up 4X5mL overnights of pSB1A3, J45100, B0034, J61003.
-ED
July 7
Lab Notes:
Glycerol stocks of overnights from July 6. Stored in -80 freezer. Miniprep from overnights started July 6. Stored in "iGEM" freezer box in -20 freezer.
-JP,JB,AL
July 8
Lab Notes:
Transform E1010 and A0500 into XL10 Gold. Plate on Kan.
Pour Kan and Amp plates. Make TBE and TAE buffer.
-ED, JP, MC, VH, CZ
July 9
Lab Notes:
Retransform E1010 and A0050 into XL10Gold cells using new plates that were poured Sunday.
-ED, VH, JG
July 10
Lab Notes:
Transformations of E1010 and A0050 were not successful. Tomorrow we will try with a new cell line and enriched media.
-ED, MC, AF, CZ
July 11
Lab Notes:
Third attempt at transforming the Kan resistant BioBricks. Transformed E1010, A0500, and pSB2K3 into XL10 Gold, XL10 Gold with enriched media (Magnesium & Glucose), and DH5a cells. Plated 100uL and the pellet remaining after taking the 100uL aliquot on Kan plates.
Restriction digest of J61003 with Xba and Spe. Run on 0.8% agarose gel. Cut out 2290 band, will gel extract tomorrow.
 E1010 XLGOLD 10 July 11
E1010 XLGOLD 10 July 11-ED, MC, NG, JG, VH, CZ
July 12
General Notes:
Calender up and running.
Meeting tonight at 7:00 in CSC 2-49.
Received Chlorobium tepidum, meeting with Dr. Jeff Fuller (with Capital Health) to discuss the use of their anaerobic chambers!! -Justin
Lab Notes:
Transformations with DH5a cells worked, but we will need to select for single colonies tonight.
Streak for single colonies of E1010, A0500, J61003.
Purification of gel slice of digested J61003.
 E1010 DH5a July 12
E1010 DH5a July 12-ED, JG, AL, JB, MC, CZ
July 13
General Notes:
Everyone who is coming tonight please be present 7:00pm sharp
Lab Notes:
Started 4X5mL overnights of E1010 and I0050 at 8:30 am. Should be ready for miniprep tonight.
-ED, JB
No growth in over nights - left these shaking in 37 incubator because they may be "slow growers"
Started new overnights of E1010, I0500, and psb2k3 (all DH5alpha) at 2200hrs incubating in the incubator by the autoclave - plates were also streaked (&labeled corresponding to the tubes)
Also made overnights for massive colonies seen on 48hr growth of E1010 in XL
Made some Kan plates
-MC, JP, JG, CZ
July 14
Schedule:
AL,JP,NK
General Notes:
If you are scheduled during the day please be in the lab at 1230hrs (PM) Don't forget that the Bio-Sci doors lock at 1900hrs so if you are trying to get in after 1900hrs call 492-2911
Things to be continued today (CZ)
1. Put LB plates in 4 degree fridge
2. Plate 20 microlitre of DH5 alpha on a kan plate as a control
3. Xba1/EcoRI double digest
NOTE: Sequential digestion is needed. Start with 1 microlitre of Xba1 and 2 microlitre of 10x Tango and incubate for an hour. Then add 1 microlitre of EcoRI and 2.5 microlitre of 10X Tango and incubate for an hour.
4. Miniprep on the colonies picked on July 13 at 10pm. They are in the shaker in the back room. Note the colonies picked were also streaked on kan plates in the 37 degree incubator. Erin's colonies are in the shaker in the front room.
If you need anything else call me(Celine). Number on the blackboard. Thank you.�
<b/>Lab Notes:</b>
JP, AL, NK, JB(?)
1. Completed Mini on E10050 (4), I0500 (2), PSB2K3 (2) samples (the two labeled Erin, are those from July 13 8:30am ON's) In DNA box -20
2. Completed sequential double restriction on J61003 promoter region. Used Xba1 and EcoR1 (see protocol). sample in DNA box -20
3. Placed Kan plates at +4
July 15
Schedule:
Jp,JB,AL
General Notes:
Options:
1. complete double restriction on newly mini'd plasmids (arabinose promoter, RFP)
2. gel purify J61003 restriction products (promoter restriction sample)
3. fundraising letters!
Lab Notes:
1. ran a gel containing the digested products of the J61003 (promoter digest), snapped photo and cut out bands for extraction/purification tomorrow
2. glycerol stocks of Chlorobium tepidum
3. finished grant application forms
- JP, AL, NG, MC
July 16
Schedule:
CZ,VH,AF
General Notes:
1. Because we didn't get into lab till late (because of locked doors), did not complete restriction on newly mini'd plasmids. Can be completed today (isolate arabinose promoter and RFP)
2. All of the restriction product (for J61003 XbaI/EcoR1) were ran and cut - no sample remaining
Lab Notes:
Completed gel extraction of E,X digested J61003. (in box in -20)
Restriction digests of E1010 (X,S) and I0500 (E,x), ran on 0.8% gel. E1010 expected a band at 680bp, I0500 expected a band at 1200 bp. Got two positive lanes for E1010: XL1 and XL2 minipreps. Cut out bands and put in -20. No positive for I0500.
-VH, ED, CZ
Quotes of the Day:
"If I had a remote, I'd change the cd."
Anonymous, in response to the skipping music on the cd player, but much to lazy to get up and change it
"I would never drive this tired. But I WILL operate chemicals."
Anonymous
July 17
Schedule:
ED,VH,AF
Lab Notes:
Gel extracted E1010 from yesterday's slices.
Mini-prep on A0500 from a culture labelled with red marker. If anyone knows what cell line is in this culture, please post.
It is I0500 and it is DH5alpha unless otherwise labelled . .. only the E1010 had both DH5alpha and XL10gold overnights -MC
Let's not do anything more with it then, because the DH5as aren't working due to them already having their own Kan resistance. - ED
Ligation of E1010 (RFP) into J61003. Used 4 different ligation conditions. These are sitting on the instructor's bench at the front of the room. These need to be transformed into XL10 Gold and plated on Amp tomorrow. Try transforming 1uL and 10uL.
-ED,JG,VH,AF
Quote of the Day
"Cancer is an urban myth."
July 18
Schedule:
CZ,MC,NG
Lab Notes:
Transformed Ligations 1,2,3,4 into XL10 Gold Competent cells
Plated 1ul,10 ul, and 200ul on AMP
Incubated @ 37celsius overnight
-MC, NG, CZ
Quote(s) of the Day:
"Asuuuuuuuuuuuum!!"
In response to the awesome; "You're wayyyy to exciting for me right now"
July 19
Meeting at 7:00, CAB 373.
Schedule:
ED,MC,JG
General Notes
New Schedule is up
check out the jar o' love @ the front :)
Lab notes
Started 8X5mL overnights to test colonies of the RFP ligation. Hopefully JB will be available to do minipreps tomorrow. Miniprep protocol is tucked in Master Book.
-ED,JG,MC
Quote of the Day
"There is a pee throw up smell it the washroom"
Reply
"Someone must be pregnant"
July 20
Schedule:
JG,ED,MC
General Notes:
There is now a Jar O' Love in the lab; Please do not drink chemicals from said jar o'love. Check it out - it's at the front on the instructors bench. It's to give shout outs for people who are rocking out and 'taking one for the team'.
Lab Notes:
Miniprep Lig3 (8 samples) overnights - JB
Made 1L LB broth (16 bottles) at the back to be put away in the sterile cupboards tomorrow
Ran Gel of JB's Minipreps after digestion with Xba and Spe. All of the colonies were positive (band at 1.2 kB).
Did some dishes - we should learn how to use the dishwasher
- MC, ED, JG
Quote(s) of the day:
"Always use protection when using the Jar O' Love"
July 21
Schedule:
JP,NK,AL
General Notes:
Since it doesn't look like Nik will be showing up, maybe just call Alex and see if it works for him.
All of the ligations of RFP into J61003 turned out. They are labelled JB July 19 1-8 in the DNA box. Transform 1 of them into XL10 Gold and plate on Amp with Tetracycline plates. You will have to add the Tet to the plates beforehand. I don't know what concentraion, but James suggested 1 ug/mL. Try plating different amounts.
The reason that I am making these suggestions is that I read that XL10Golds are Tet resistant, so we can maybe induce the expression of RFP with the Tet because it is under the Tet promotor and then we can see the RFP.
As always, call me if you need anything.
-ED
Lab Notes:
1. got into building
2. waited for peers - no shows
3. tried to break into room by climbing through roof (cement goes all the way up)
4. went home... will complete work tomorrow - sorry i'll get swipe access asap...
July 22
Schedule:
JP,AL,NG
General Notes:
How does 7:00pm sound?
Laboratory Notes:
Transformed XL10 gold cells with J61003 (with RFP ligated in). Split cell aliquot into two 50microL aliquots and added 1microL DNA from ligation sample 1 and from ligation sample 4. Covered AMP plates with 50microL 1microg/ml tet, spread, let sit. Plated (1) 50microL sample and (1) 5microL + 45microL LB onto Tet/Amp plates for #'s 1&4. They are in 37C incubator. The remaining transformed cells are in +4C labeled July 22.
Serious Quote of the Month
Faust
Then shall I see, with vision clear,
How secret elements cohere,
And what the universe engirds,
And give up huckstering with words
-Johann Wolfgang von Goethe
July 23
Schedule:
VH,CZ,JG
Laboratory Notes:
1. Add more Tet on ligation plates from July 22. Hopefully red colonies will appear!
2. Transformation of XL10Gold with
- Butanol CoA dehydrogenase (1 in 1000 dilution)
- Butenoyl CoA dehydrogenase (1 in 1000 dilution)
- J23018 (RFP positive control)
20 and 100 microlitres of transformed culture plated on Amp plate and incubate at 37 degrees
The leftover mixture is stored in 4 degree fridge in case of no growth.
3. Repeate overnight ligation from July 22. The exact amounts of insert, vector etc. are on the blackboard.
For tomorrow:
1. Pick colonies for transformed plates.
2. Plate ligation mixtures with appropriate amount of Tet.
3. Observe red colonies from the July 22 ligation plates if the cells are still alive.
Memorable Quote:
Don't force it. Let it come out naturally.
to the top
July 24
Schedule:
AF,ED,NG
Lab Notes:
Transformed I0500 (arabinose promotor) into HB101 competent cells. Plated 2, 20, 200 uL on Kan.
Transformed ligations from yesterday into XL10 gold. Plated 100 uL on Amp.
RFP control from yesterday worked, but sadly our ligations are not expressing any RFP. It was still exciting to see red control cells. It seems as if the Tet promotor is induced at a low level without any Tet.
Started 4X5ml overnights of enoyl coA hydratase and butyryl coa DH. There seems to be some confusion with the naming of these proteins. These are the names that I have used in the BioBricks. We will be able to tell if they are correctly labelled when we do the digests. Enoyl coa hydratase is about 840 bp and butyryl coa DH is about 1200 bp.
Note on agarose gels: Use only the wide combs (8 lanes) because we are having to many problems with the narrow lanes. This may mean that you will need to pour multiple gels (oh my). Also, try and run two ladders per gel until we get the ladder problem sorted out. Also, make sure that you zoom in on the gel when taking a picture.
Note on Tet: should be stored 12.5 mg/mL in 50% ethanol, 50% water solution. This is a 1000X solution. Keep this in mind when using Tet again.
-ED, AF, James
July 25
Schedule:
Jp,MC,VH
General Notes:
Use of wide combs- 5microL of sample works well with 3.5microL of ladder. Make sure you stir up the ladder before use!
Lab Notes:
Completed minipreps of enoylcoa hydratase and butyryl coa dh. They are in the new box with the green tape. I think that there are two types of digests we should be doing: one to make sure we have the right stuff (digest with EcoR1 and Pst1) and one to clone in the RBS. To clone in the RBS, cut B0034 with Eco and Spe and cut the enoyl coa hydratase and butyryl coa dh with Eco and Xba.
Check to see if any colonies have grown on I0500 plates in incubator. There weren't any visible on Adam's transformations this morning. James also transformed some this morning, so they may be hard to see. If there are colonies, start overnights and see if Jori can do minipreps tomorrow. If Jori can't make it, get ahold of me and I can. AND if there are no colonies, just post here and I'll go check the plates in the morning.
I also forgot to start glycerol stocks of our enoly coa hydratase and butyryl coa dh. If you guys could restart some overnights from the plates in the fridge so I can do it tomorrow that would be great.
I had to rush to work this morning and didn't get any of this written in the lab book. So you guys can make fun of me or whatever. I guess I belong in the Science Hall of Shame.
-ED
Enny = Enoly CoA Hydratase
Benny = Butytyl CoA DH
Checked plates
- Adam - no growth: parafilmed and put in 4 fridge
- James'? J23018: red colonies parafilmed and put in 4 fridge (was this the control?)
Overnights of Benny & Enny
- 3x 5mlLB each
Restrictions
- B0034 with SPE & ECO
- Enny with Xba & ECO
-Benny with Xba & ECO
These are stored in the -20 freezer
- MC, NK, VH, NG, JP
For Tomorrow:
- Glycerol stocks of Enny & Benny (these colonies are likely different from the transformations as it was not marked out which colonies were picked for transformations)
- Gels for restrictions of B0034, Enny & Benny
- cut out the bands; gel extractions? (if there is time)
July 26
Meeting at 7:00, Room CAB 373.
Schedule:
AL,VH,ED
Lab Book
Ran gel of "Enny", "Benny" and Boo IV against 1 kb ladder.
Cleared up glasswares in the autoclave.
Parafilm all unparafilmed plates.
Checked out red fluorescence of J23018.
Attempted working on fundraising letters (?)
-ED, VH, AL, JG, MC, AF, Doug
Favourite "F" word of the day:
fulcrum
July 27
Schedule:
JP,MC,NK
The theme is Foul music Friday - bring any bad music you have (or embarassed that you have in your collection)
General Notes:
1. This is whats on the agenda for this evening:
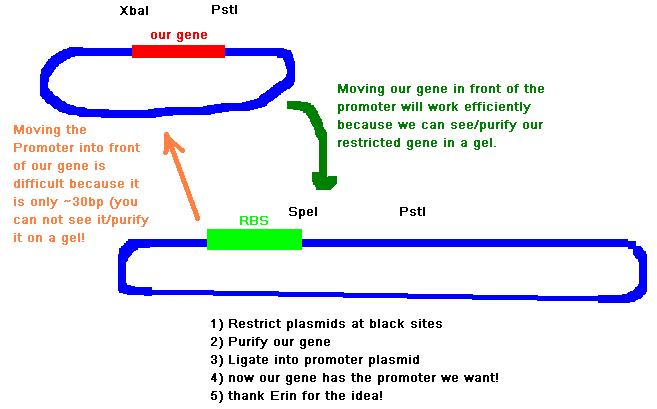
Lab book:
Digest Enny(1,4), Benny (1,2) B0034(2,4) with PST, XBA, SPE
Enny with PST and XBA
Benny with PST and XBA
B0034 with PST and SPE
O/N of Benny and Enny glycerol stock (3 each)
Note the 4 degree fridge at the back is out of commision. All iGEM materials have been moved to the room where we take pictures of gels. The door labelled IGEM. Nik is here on saturday and Celine can show on sunday.
- JG, NK, MC, JP, and James (cos he loves us)
Jame's Fun Tip of the Day:
If you want to run a gel for a shorter period of time ad the EtBR to the gel rather than the buffer. You can add ~4ul to the gel rather than 10ul to the buffer.
July 28
Schedule:
CZ,JB,NK
Meeting @ 1300hrs - MC on behalf of CZ
General Notes:
To Do:
- Glycerol stocks of Enny & Benny from the O/N
- Gels of Enny, Benny & B0034 (put the EtBr in the gel)
- Ligations of Enny+Benny into B0034 with T4 ligase; leave O/N in incubator at the back room (near the phone) set at 13degrees celsius
Lab Book
Finished making glycerol stocks of Enny and Benny from the O/N. Followed Erin's protocols except the Flash Freeze step.
Ran agarose gel of Benny, Enny, b0034 digest.
No DnA ladder (NEVER DO IT AGAIN).
Ran at 90V for 20 Min, run longer for next time.
Cut Benny band (1200BP) and B0034, need to redigest Enny tomorrow
Gel extration using QIAGEN kit
Ligation , control only with ligase,
Stored at 13 degrees celsius overnight
July 29
Schedule:
CZ,AF,NG
As agreed on Thursday meeting at noon or 12
General Notes:
1. Transformation of ligation (Benny and B0034( into XL10 Gold
on Amp plates; incubate at 37 degrees. Hopefully we have colonies to pick tomorrow.
2. Double digest of Enny (Pst/Xba) and B0034 (Pst/Spe) repeated; Enny band (800bp) and B0034 (2.7kb) bands cut out and purified; overnight ligation mixture at 13 degree incubator at the back of the room
For tomorrow:
1. Pick colonies of Benny and B0034
2. Transformation of Enny and B0034
July 30
Schedule:
VH,JP,JG,
Lab SHTUFF
1- started overnights on 10 different colonies benny colonies(see lab book for details)
2- transformed new enny's and one boo34 control (plates at 37 degrees)
3- made new amp
4- sent out sponsorship letters!
Sports quote of the Day
You train to play good, you hope to play good, I think we played like bad@zz'z
July 31
Schedule:
AF,JP,MC
Laborious Notes:
1:Restriction of Boo34 with SpeI and PstI (Now we have tons of stock restricted plasmid just incase and we are SURE that these have been restricted to the maximus!
2:mini preps on all of the 'Benny in boo34' overnights along with glycerol stocks of each
3:poured a fancy gel for tomorrows usage (EtBr in gel)
4:started overnites on 'Enny in Boo34' (plates in 4 degrees)
5:transformed TWO NEW GENES BuOH DH ("Buddy") and BBbhBu_CoA DH ("Betty") into golden bacteria (at 37 degrees)
5a: Buddy & Betty are in 1.5ml tubes in the 4degree fridge incase the plates do not show growth.
Rule of the day
No Touching.
Fact of the day
Vortexing after lysis is not like getting a bad haircut. Hair grows back.
UofA iGEM Home
To June 2007
To August 2007