Alberta/Calender/October
From 2007.igem.org
(→October 26) |
|||
| (69 intermediate revisions not shown) | |||
| Line 80: | Line 80: | ||
Back to [[Alberta|UofA iGEM Home]]<br> | Back to [[Alberta|UofA iGEM Home]]<br> | ||
| - | == October 1 == | + | == October 1 == <br> |
| + | JG<br> | ||
| + | Miniprep J61003+Enny and Benny+J61003<br> | ||
| + | Minis are in -20<br><br> | ||
| + | ML<br> | ||
| + | Brought the tubes labelled "sequencing rxns" up to MBSU<br> | ||
| + | Also brought some XBA 1 from fermentas freezer since we ran out<br> | ||
| + | Digests JG's miniprep with Xbal and PST. Ran out of out of XBA during digests, which meant that EJ 4,5,6only digested with XBA for 35 min<br> | ||
| + | Colony O/N of I0500/ J61003 and Buddy/J61003<br> | ||
| + | |||
| + | |||
| Line 88: | Line 98: | ||
== October 2 == | == October 2 == | ||
| + | VH-1PM<br> | ||
| + | No Kan plates therefore made kan plates<br> | ||
| + | On COuntertop<br> | ||
| + | Miniprepped ML's overnights from OCt 1<br> | ||
| + | Lysis solution is a no go<br> | ||
| + | Started new O/N of previows overnights for tomorrow<br> | ||
| + | No more LB<br> | ||
| + | WM is Wayne Materi (a team advisor) in the following. | ||
| + | |||
| + | WM- | ||
| + | Assembling all the individual genes into an operon in order of their role in the butanoate pathway from KEGG. | ||
| + | We will start with I725021 (RBS + B-hydroxy butyryl coA dehydrogenase in the B0034 plasmid - pSB1A2)then insert I725022 (RBS + Enoyl-coa hydratase), then I725023 (RBS + Butyryl coa Dehydrogenase), then I725024 (RBS + Butyraldehyde dehydrogenase), and finally I725025 (RBS + Butanol dehydrogenase). After the operon is constructed and verified, we will insert the Arabinose promoter from I0500 5' to all the genes. | ||
| + | |||
| + | Digest I725021/SpeI+PstI and gel purify ~3kb band | ||
| + | Digest I725022/SpeI+PstI and gel purify ~1.2kb band | ||
| + | |||
| + | Ligate (Got 16 colonies vs. 10 for negative ctl). | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| - | == October 3 == | + | == October 3 == <br> |
| + | |||
| + | MC - 800hrs <br> | ||
| + | Autoclaved 2 bottosl of Ependorf tupes- to be picked up from G308<br> | ||
| + | Transform THolase into XL10 gold plates<br> | ||
| + | Miniprep of 10500+J61003 O/N<br> | ||
| + | ML<br> | ||
| + | Digest of 10500+J61003 with ECORI and XBA<br> | ||
| + | Housekeeping complete<br> | ||
| + | Note to Justin: Samples to sequence are in -20 labelled "Justin! Sequence me"<br> | ||
| + | CZ - 7:00pm<br> | ||
| + | Ran gel of I0500/J61003<br> | ||
| + | It looks like I05oo is in J61003 but have to confirm with Justin or Michelle or Erin. <br> | ||
| + | <b>NB: please note the lab is unavailable EVERY wednesday from 1400-1700hrs</b><br> | ||
| Line 100: | Line 140: | ||
== October 4 == | == October 4 == | ||
| + | NK - 930<br> | ||
| + | VH - 2pm<br> | ||
| + | Cleaned up 37 degree<br> | ||
| + | Moved 30th plates of I0500+JG and Buddy+j6 to 4 degree fridge<br> | ||
| + | Disposed of really disgusting plates<br> | ||
| + | Got ride of I0500 ovrnights that hve been on shaker for a wek now<br> | ||
| + | CHecke thoolase kan plates<br> | ||
| + | Showing blue dots so far and af we white doets<br> | ||
| + | Left plates in 37 celsious room<br><br> | ||
| + | JP<br> | ||
| + | Sequencing of 3 primer of DBS<br> | ||
| + | Ligate I0500 eco/spe into J61003 (eco/xba)<br> | ||
| + | |||
| + | WM - Primers for colony PCR and sequencing are as follows: <br> | ||
| + | Primer 1 (3' end of I725021) CTGGTTGGCTGGGTCGTAAATCC <br> | ||
| + | Primer 2 (3' end of I725022) GACGCTATGACCGCTTTCATCG <br> | ||
| + | Primer 3 (3' end of I725023) CTACGAAGGTACCTCCGAAGTTC <br> | ||
| + | Primer 4 (3' end of I725024) CGCTGATCTCCGAACTGAAAGAC <br> | ||
| + | Primer 5 (3' end of I725025) CTGCGTCCGGTTAACGCTTCC <br> | ||
| + | VF and VR as per BioBricks <br> | ||
| + | <br> | ||
| + | Performed Colony PCR on 10 candidates for BuOP1 (I725021 + I725022) using Primer1 and VR (expect 1063 bp band if good) <br> | ||
| + | <br> | ||
| + | Expect | ||
| + | [[Image:UVP00153annot.jpg]]<br> | ||
| + | <br> | ||
| + | Colonies 1 and 8 look good. Sequence with Primer 1. <br> | ||
| Line 108: | Line 175: | ||
== October 5 == | == October 5 == | ||
| + | JG/MC - 800hrs <br> | ||
| + | Ran gel of i0500 and buddy<br> | ||
| + | Digest #4 J61003/I0500 Digest Eco/xba with Pst to drop out of GFP<br> | ||
| + | Ran gel of I0500J61003 eco/xba<br><br> | ||
| + | ML<br> | ||
| + | Gel completd and took photos<br> | ||
| + | Buddy and I0500 digested<br> | ||
| + | Extracted gel<br> | ||
| + | Labelled I0500 purify Oct5 and Buddy Purify oct 5<br><br> | ||
| + | CZ - Sorry I can't make it for personal reasons. | ||
| + | |||
| + | WM - BuOP1.1 and BuOP1.8 both sequenced good from nt 1096 to 1816. | ||
| + | |||
| + | Also ran a verifying digest on BuOP1 O/Ns | ||
| + | |||
| + | BuOP1/XbaI + SpeI (expect 2149 and 1709 bp if good) | ||
| + | |||
| + | [[Image:UVP00157annot.jpg]] | ||
| + | |||
| + | BuOP1.1 and .8 both look good. | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 115: | Line 202: | ||
== October 6 == | == October 6 == | ||
| + | ED 9:00<br> | ||
| + | Made a to do list for the day<br> | ||
| + | |||
| + | NK 2pm<br> | ||
| + | Ligations of I0500 purify oct 5 and J61003<br> | ||
| + | Left on bench with green tape labelled "ligations oct6"<br> | ||
| + | Digest Boo34 with s,p<br> | ||
| + | Digests in freezer labelled "digestions B0034 S,P"<br> | ||
| + | |||
| + | WM - Make BuOP2 by inserting I725023 into BuOP1 | ||
| + | |||
| + | Digests: BuOP1/SpeI+PstI (gel purify ~3.8kb band) | ||
| + | I725023/XbaI+PstI (gel purify ~850bp band - already have) | ||
| + | |||
| + | [[Image:UVP00158annot.jpg]] | ||
| + | Cutout, bands, quantitated DNA and ligated -> Plate 150 ul on LB+Amp | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 121: | Line 224: | ||
== October 7 == | == October 7 == | ||
| + | ED 9:00 | ||
| + | NG 12:00 | ||
| + | WM - did 8 O/Ns and minipreps | ||
| + | |||
| + | Verify with XbaI+SpeI digest (expect 2149+2916bp bands if good) | ||
| + | |||
| + | [[Image:UVP00160annot.jpg]] | ||
| + | |||
| + | All but 4 and 8 look good. Sequence BuOP2.1, 2.2, 2.5, 2.6 with Primer 2 | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 128: | Line 240: | ||
== October 8 == | == October 8 == | ||
| - | + | WM - BuOP2.1, 2.2, 2.5, 2.6 all sequenced good. | |
| Line 134: | Line 246: | ||
== October 9 == | == October 9 == | ||
| + | NK<br> | ||
| + | Loaded gel with digets from october 6, boo34 S,P<br> | ||
| + | Put the ligations from oct6 in the freezer with the tape in tray #3<br> | ||
| - | + | VH<br> | |
| - | + | Gel extrations B0034 sp1 and boo34 sp2<br> | |
| Line 142: | Line 257: | ||
== October 10 == | == October 10 == | ||
| + | JP?<br> | ||
| + | Tholase PCR<br><br> | ||
| + | MC<br> | ||
| + | Ligated Buddy oct 5 into Boo34 digest oct 6<br> | ||
| + | Left on bench<br> | ||
| + | WM - Make BuOP3 by inserting I725024 into BuOP2 | ||
| + | Digests: BuOP2/SpeI+PstI (expect 5047bp -> gel purify) | ||
| + | I725024/XbaI+PstI (expect 2149bp and 2642bp - gel purify larger band)Not shown | ||
| + | |||
| + | [[Image:UVP00161annot.jpg]] | ||
| + | |||
| + | Cutout all four bands and gel purify. | ||
| + | I725024 was digested and gel-purified seperately. | ||
| + | Quantitation of bands showed ~5pmol/ul for BuOP2/SpeI+PstI digests and ~7pmol/ul for I725024/XbaI+PstI digests. | ||
| + | |||
| + | Ligations (fast tracking construction). Plate 150ul on LB+Amp | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
== October 11 == | == October 11 == | ||
| + | JP?<br> | ||
| + | PCR thiolase<br> | ||
| + | Transformed Buddy in Boo 1+2 into competentent cells, plated on AMP+ plates | ||
| + | |||
| + | WM - Do Colony PCR on 7 colonies (from very few transformants) with Primer 4 and VR (expect ~240bp if good). | ||
| + | [[Image:UVP00165annot.jpg]] | ||
| + | Didn't work very well. Try confirming by digest. | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 155: | Line 293: | ||
== October 12 == | == October 12 == | ||
| + | MC, JG @ 800hrs<br> | ||
| + | Ran gel of PCR products<br> | ||
| + | No growth on the buddy in Boo transformation<br> | ||
| + | Religations of buddy into boo but couldnt find Buddy therefore religations halted | ||
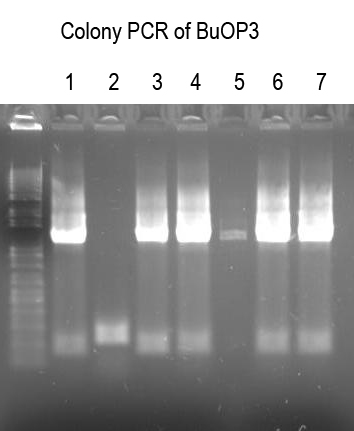
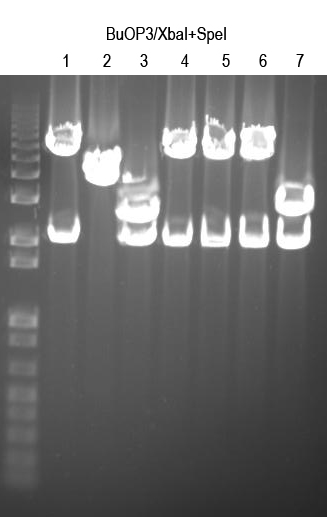
| + | WM - Verify BuOP3 by digest with XbaI+SpeI (expect 2.1 and 5.6kb bands if good) | ||
| + | |||
| + | [[Image:UVP00166annot.jpg]] | ||
| + | |||
| + | Sequence 1, 4, 5, 6 with Primer 3 to confirm | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
== October 13 == | == October 13 == | ||
| + | ML<br> | ||
| + | Religated buddy and Boo<br> | ||
| + | Note: when free of tasks work on poster, or presentation or wiki or call justin or erin | ||
| + | WM - BuOP3.4 and 3.5 sequenced good. Due to time considerations we will call BuOP3.4 part I725099 and submit this. | ||
| Line 166: | Line 317: | ||
== October 14 == | == October 14 == | ||
| + | JG, MC<br> | ||
| + | Retransform Buddy and Boo34<br> | ||
| + | Transform tholase<br><br> | ||
| + | |||
| + | JP<br> | ||
| + | Restriction of thiolase with ECO/PST<br> | ||
| + | Ran gel<br> | ||
| + | Transform rom Bud "2" oct 12<br> | ||
| + | |||
| Line 172: | Line 332: | ||
== October 15 == | == October 15 == | ||
| + | Re-digestions for re-ligations of boo34 and buddy<br> | ||
| + | Digest boo34 s/p<br> | ||
| + | Ran gel<br> | ||
| + | Extract tholase from oct 14 into tube labelled "THiolase band EX"<br> | ||
| + | Updated wiki<br><br> | ||
| - | + | To do:<br> | |
| + | Extract gel<br> | ||
| + | Ligate with buddy<br> | ||
| + | Run ligation on gel<br> | ||
| + | Extract<br> | ||
| + | Transform<br> | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 179: | Line 349: | ||
== October 16 == | == October 16 == | ||
| + | NK- Digested B0034 with Spe and PST. | ||
| + | |||
| + | -Ran a gel of restriction | ||
| + | |||
| + | -Extracted Thiolase from gel on previous day. | ||
| + | |||
| + | VH and AL - Took a picture of gel | ||
| + | |||
| + | -Cut out and purified four bands of B0034 from previous digestion | ||
| + | |||
| + | JP- Ligate B0034 with Buddy | ||
| + | |||
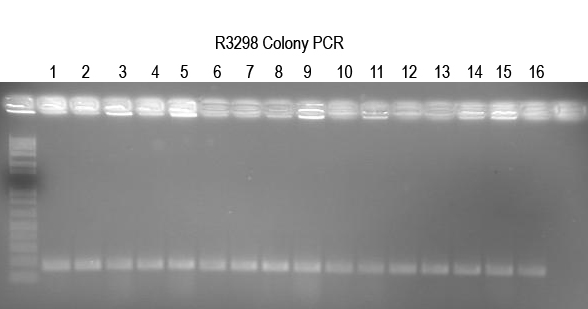
| + | WM - Verifying I725025 a.k.a. Buddy in Boo) | ||
| + | The ligation and transformation already seem to have been done and produced many colonies. So I will do colony PCR with Primer 5 and VR (expect 250 bp) to confirm. | ||
| + | |||
| + | [[Image:UVP00169annot.jpg]] | ||
| + | |||
| + | All 16 colonies tested look good. Setup 12 O/Ns to verify by digest. | ||
| Line 184: | Line 372: | ||
== October 17 == | == October 17 == | ||
| + | |||
| + | |||
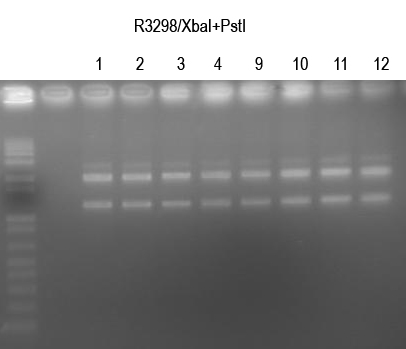
| + | WM verify I725025 by /XbaI+SpeI digest (expect 2149+1.2kb bands) | ||
| + | |||
| + | [[Image:UVP00170annot.jpg]] | ||
| + | |||
| + | All looked good. Sequence 1, 2, 9, 10. | ||
| Line 191: | Line 386: | ||
== October 18 == | == October 18 == | ||
| + | VH - Transformed B0034, Buddy ligations from yesterday and plated them on Amp plates | ||
| + | WM - I725025 sequences all good. Use #1. | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 214: | Line 411: | ||
== October 22 == | == October 22 == | ||
| + | I'll be there at 3-NK | ||
| + | ML - Gel purified Boo-Thiolase | ||
| + | -Digested B0034 with ECO and PST | ||
| + | |||
| + | -Ligated Thiolase with B0034 | ||
| + | |||
| + | WM - Add arabinose promoter in front of individual genes so that each protein can be seperately expressed and purified using the His-tags we introduced. | ||
| + | |||
| + | Arabinose promoter (+AraC repressor) comes frmo I0500. | ||
| + | |||
| + | Digests: | ||
| + | I725021/EcoRI+XbaI | ||
| + | I725022/EcoRI+XbaI | ||
| + | I725023/EcoRI+XbaI | ||
| + | I725024/EcoRI+XbaI | ||
| + | I725025/EcoRI+XbaI | ||
| + | I0500/EcoRI+SpeI | ||
| + | |||
| + | [[Image:UVP00173annot.jpg]] | ||
| + | |||
| + | Note: I0500 concentration is quite low. Probably need to grow it up O/N then induce O/N with IPTG after that. | ||
| + | |||
| + | Setup ligations anyway. | ||
[[Alberta/Calender/October#October|to the top]] | [[Alberta/Calender/October#October|to the top]] | ||
| Line 221: | Line 441: | ||
== October 23 == | == October 23 == | ||
| + | AL and NK - Transformed B0034 and Thiolase into XL10 Gold Cells. | ||
| + | |||
| + | WM - very few colonies from ligation | ||
| + | Did 1 O/N from each kind of ligation/transformation for digest verification. | ||
| Line 227: | Line 451: | ||
== October 24 == | == October 24 == | ||
| + | WM - Digest O/Ns of arabinose + individual genes for I725021, 022, 024, 025 | ||
| + | Digest with /XbaI+SpeI | ||
| + | |||
| + | Expected sizes (in addition to common 2149bp band): | ||
| + | I725021 - 2100bp | ||
| + | I725022 - 2400bp | ||
| + | I725024 - 3839bp | ||
| + | I725025 - 2435bp | ||
| + | |||
| + | [[Image:UVP00175annot.jpg]] | ||
| + | |||
| + | First and last ligation looked like they worked. We will try to do this again. | ||
| Line 239: | Line 475: | ||
== October 26 == | == October 26 == | ||
| + | Many thiolase in B0034 colonies. Started overnights for mini and digests tomorrow. | ||
| + | jp- | ||
Latest revision as of 01:45, 27 October 2007
| ||||||||||||||||||||||||||||||||||||||||||||||||||
To September 2007
To November 2007
Back to UofA iGEM Home
== October 1 ==
JG
Miniprep J61003+Enny and Benny+J61003
Minis are in -20
ML
Brought the tubes labelled "sequencing rxns" up to MBSU
Also brought some XBA 1 from fermentas freezer since we ran out
Digests JG's miniprep with Xbal and PST. Ran out of out of XBA during digests, which meant that EJ 4,5,6only digested with XBA for 35 min
Colony O/N of I0500/ J61003 and Buddy/J61003
October 2
VH-1PM
No Kan plates therefore made kan plates
On COuntertop
Miniprepped ML's overnights from OCt 1
Lysis solution is a no go
Started new O/N of previows overnights for tomorrow
No more LB
WM is Wayne Materi (a team advisor) in the following.
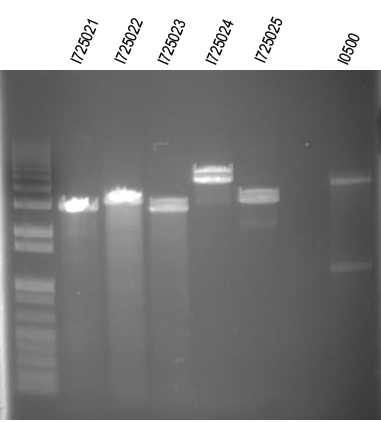
WM- Assembling all the individual genes into an operon in order of their role in the butanoate pathway from KEGG. We will start with I725021 (RBS + B-hydroxy butyryl coA dehydrogenase in the B0034 plasmid - pSB1A2)then insert I725022 (RBS + Enoyl-coa hydratase), then I725023 (RBS + Butyryl coa Dehydrogenase), then I725024 (RBS + Butyraldehyde dehydrogenase), and finally I725025 (RBS + Butanol dehydrogenase). After the operon is constructed and verified, we will insert the Arabinose promoter from I0500 5' to all the genes.
Digest I725021/SpeI+PstI and gel purify ~3kb band Digest I725022/SpeI+PstI and gel purify ~1.2kb band
Ligate (Got 16 colonies vs. 10 for negative ctl).
== October 3 ==
MC - 800hrs
Autoclaved 2 bottosl of Ependorf tupes- to be picked up from G308
Transform THolase into XL10 gold plates
Miniprep of 10500+J61003 O/N
ML
Digest of 10500+J61003 with ECORI and XBA
Housekeeping complete
Note to Justin: Samples to sequence are in -20 labelled "Justin! Sequence me"
CZ - 7:00pm
Ran gel of I0500/J61003
It looks like I05oo is in J61003 but have to confirm with Justin or Michelle or Erin.
NB: please note the lab is unavailable EVERY wednesday from 1400-1700hrs
October 4
NK - 930
VH - 2pm
Cleaned up 37 degree
Moved 30th plates of I0500+JG and Buddy+j6 to 4 degree fridge
Disposed of really disgusting plates
Got ride of I0500 ovrnights that hve been on shaker for a wek now
CHecke thoolase kan plates
Showing blue dots so far and af we white doets
Left plates in 37 celsious room
JP
Sequencing of 3 primer of DBS
Ligate I0500 eco/spe into J61003 (eco/xba)
WM - Primers for colony PCR and sequencing are as follows:
Primer 1 (3' end of I725021) CTGGTTGGCTGGGTCGTAAATCC
Primer 2 (3' end of I725022) GACGCTATGACCGCTTTCATCG
Primer 3 (3' end of I725023) CTACGAAGGTACCTCCGAAGTTC
Primer 4 (3' end of I725024) CGCTGATCTCCGAACTGAAAGAC
Primer 5 (3' end of I725025) CTGCGTCCGGTTAACGCTTCC
VF and VR as per BioBricks
Performed Colony PCR on 10 candidates for BuOP1 (I725021 + I725022) using Primer1 and VR (expect 1063 bp band if good)
Expect
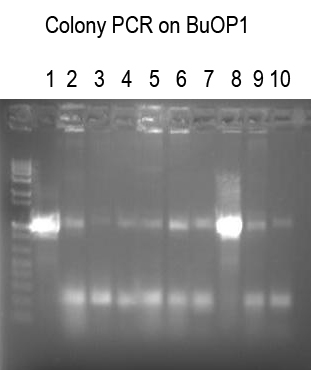
Colonies 1 and 8 look good. Sequence with Primer 1.
October 5
JG/MC - 800hrs
Ran gel of i0500 and buddy
Digest #4 J61003/I0500 Digest Eco/xba with Pst to drop out of GFP
Ran gel of I0500J61003 eco/xba
ML
Gel completd and took photos
Buddy and I0500 digested
Extracted gel
Labelled I0500 purify Oct5 and Buddy Purify oct 5
CZ - Sorry I can't make it for personal reasons.
WM - BuOP1.1 and BuOP1.8 both sequenced good from nt 1096 to 1816.
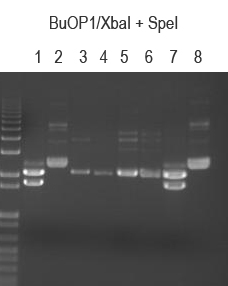
Also ran a verifying digest on BuOP1 O/Ns
BuOP1/XbaI + SpeI (expect 2149 and 1709 bp if good)
BuOP1.1 and .8 both look good.
October 6
ED 9:00
Made a to do list for the day
NK 2pm
Ligations of I0500 purify oct 5 and J61003
Left on bench with green tape labelled "ligations oct6"
Digest Boo34 with s,p
Digests in freezer labelled "digestions B0034 S,P"
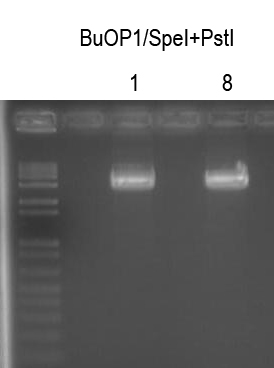
WM - Make BuOP2 by inserting I725023 into BuOP1
Digests: BuOP1/SpeI+PstI (gel purify ~3.8kb band)
I725023/XbaI+PstI (gel purify ~850bp band - already have)
Cutout, bands, quantitated DNA and ligated -> Plate 150 ul on LB+Amp
October 7
ED 9:00
NG 12:00
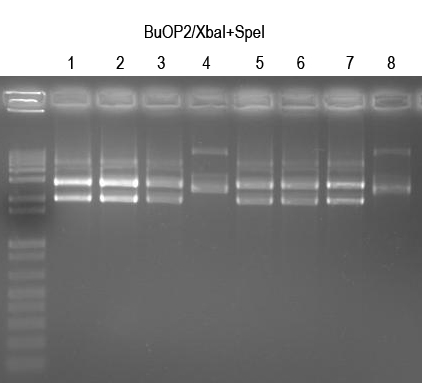
WM - did 8 O/Ns and minipreps
Verify with XbaI+SpeI digest (expect 2149+2916bp bands if good)
All but 4 and 8 look good. Sequence BuOP2.1, 2.2, 2.5, 2.6 with Primer 2
October 8
WM - BuOP2.1, 2.2, 2.5, 2.6 all sequenced good.
October 9
NK
Loaded gel with digets from october 6, boo34 S,P
Put the ligations from oct6 in the freezer with the tape in tray #3
VH
Gel extrations B0034 sp1 and boo34 sp2
October 10
JP?
Tholase PCR
MC
Ligated Buddy oct 5 into Boo34 digest oct 6
Left on bench
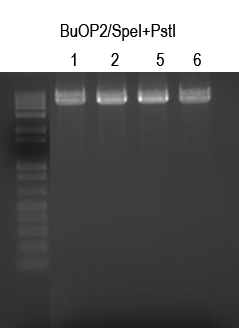
WM - Make BuOP3 by inserting I725024 into BuOP2
Digests: BuOP2/SpeI+PstI (expect 5047bp -> gel purify)
I725024/XbaI+PstI (expect 2149bp and 2642bp - gel purify larger band)Not shown
Cutout all four bands and gel purify. I725024 was digested and gel-purified seperately. Quantitation of bands showed ~5pmol/ul for BuOP2/SpeI+PstI digests and ~7pmol/ul for I725024/XbaI+PstI digests.
Ligations (fast tracking construction). Plate 150ul on LB+Amp
October 11
JP?
PCR thiolase
Transformed Buddy in Boo 1+2 into competentent cells, plated on AMP+ plates
WM - Do Colony PCR on 7 colonies (from very few transformants) with Primer 4 and VR (expect ~240bp if good).
Didn't work very well. Try confirming by digest.
October 12
MC, JG @ 800hrs
Ran gel of PCR products
No growth on the buddy in Boo transformation
Religations of buddy into boo but couldnt find Buddy therefore religations halted
WM - Verify BuOP3 by digest with XbaI+SpeI (expect 2.1 and 5.6kb bands if good)
Sequence 1, 4, 5, 6 with Primer 3 to confirm
October 13
ML
Religated buddy and Boo
Note: when free of tasks work on poster, or presentation or wiki or call justin or erin
WM - BuOP3.4 and 3.5 sequenced good. Due to time considerations we will call BuOP3.4 part I725099 and submit this.
October 14
JG, MC
Retransform Buddy and Boo34
Transform tholase
JP
Restriction of thiolase with ECO/PST
Ran gel
Transform rom Bud "2" oct 12
October 15
Re-digestions for re-ligations of boo34 and buddy
Digest boo34 s/p
Ran gel
Extract tholase from oct 14 into tube labelled "THiolase band EX"
Updated wiki
To do:
Extract gel
Ligate with buddy
Run ligation on gel
Extract
Transform
October 16
NK- Digested B0034 with Spe and PST.
-Ran a gel of restriction
-Extracted Thiolase from gel on previous day.
VH and AL - Took a picture of gel
-Cut out and purified four bands of B0034 from previous digestion
JP- Ligate B0034 with Buddy
WM - Verifying I725025 a.k.a. Buddy in Boo) The ligation and transformation already seem to have been done and produced many colonies. So I will do colony PCR with Primer 5 and VR (expect 250 bp) to confirm.
All 16 colonies tested look good. Setup 12 O/Ns to verify by digest.
October 17
WM verify I725025 by /XbaI+SpeI digest (expect 2149+1.2kb bands)
All looked good. Sequence 1, 2, 9, 10.
October 18
VH - Transformed B0034, Buddy ligations from yesterday and plated them on Amp plates
WM - I725025 sequences all good. Use #1.
October 19
October 20
October 21
October 22
I'll be there at 3-NK
ML - Gel purified Boo-Thiolase
-Digested B0034 with ECO and PST
-Ligated Thiolase with B0034
WM - Add arabinose promoter in front of individual genes so that each protein can be seperately expressed and purified using the His-tags we introduced.
Arabinose promoter (+AraC repressor) comes frmo I0500.
Digests:
I725021/EcoRI+XbaI
I725022/EcoRI+XbaI
I725023/EcoRI+XbaI
I725024/EcoRI+XbaI
I725025/EcoRI+XbaI
I0500/EcoRI+SpeI
Note: I0500 concentration is quite low. Probably need to grow it up O/N then induce O/N with IPTG after that.
Setup ligations anyway.
October 23
AL and NK - Transformed B0034 and Thiolase into XL10 Gold Cells.
WM - very few colonies from ligation Did 1 O/N from each kind of ligation/transformation for digest verification.
October 24
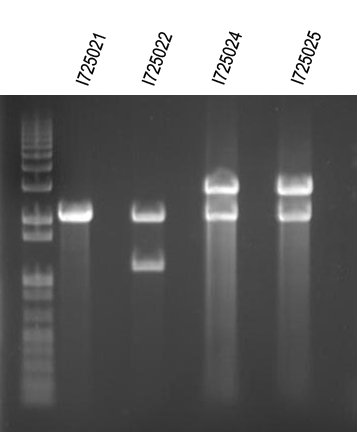
WM - Digest O/Ns of arabinose + individual genes for I725021, 022, 024, 025 Digest with /XbaI+SpeI
Expected sizes (in addition to common 2149bp band):
I725021 - 2100bp
I725022 - 2400bp
I725024 - 3839bp
I725025 - 2435bp
First and last ligation looked like they worked. We will try to do this again.
October 25
October 26
Many thiolase in B0034 colonies. Started overnights for mini and digests tomorrow. jp-
October 27
October 28
October 29
October 30
UofA iGEM Home
To September 2007
To November 2007