BerkiGEM2007Present6
From 2007.igem.org
<< Back to UC Berkeley iGEM 2007
Human Practices
Human Practices examines the way synthetic biology might inform human security, health, and welfare through the new objects that synthetic biology brings into the world. It also observes the ways in which economic, political, and cultural forces may shape the development of synthetic biology.
Summer Work
I focused my investigation on the patentability of Bacto Blood where its parts are in an open source forum given that there are existing patents on oxygen-based therapeutics. If it were possible to patent Bacto Blood, how could my team obtain patent protection for their product? Bacto Blood is patentable despite having its parts listed an open source forum. However, patentability of Bacto Blood may be complex. Two questions that read on Bacto Blood’s patentability are:
1. What aspects of Bacto Blood are patentable, the individual part(s), the part(s) as put together into the whole, or the applications made possible by the part(s)?
2. What starts the timeline for patenting Bacto Blood: when the part is put on the registry or when the applications of the part is made public?
Novelty and Non Obvious
There are existing patents relating to different types of oxygen therapeutics. These patents include PFC compunds’ as oxygen therapeutics and methods to maximize the production yields of hemoglobin using Escherichia coli expression systems. The application of Bacto Blood’s E. coli must be both novel and non-obvious over the prior art. The novelty and nonobviousness of Bacto Blood relative to other oxygen therapeutics lies in the expression of hemoglobin in an E. coli system that is genetically engineered to be safe in vivo human therapy. Bacto Blood is novel because the team created biological parts that can be used to suppress the normal replication cycle of E. coli so that it does not cause sepsis in the human body. The “aseptic” bacteria were then combined with other biological parts created by different team members. The parts and the different devices generated by the combinations of parts such as, (an oxygen carrier, a controller, a self-destruct mechanism, and a freeze drying component) were constructed and inserted into the “aseptic” E. coli.
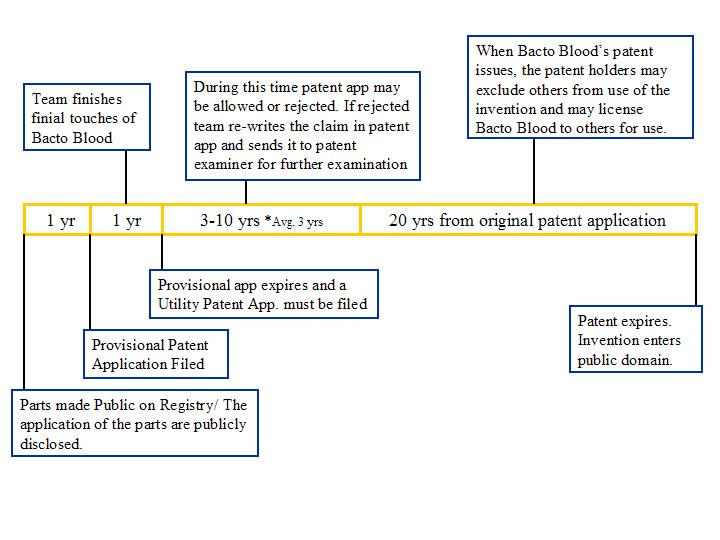
Patent application timeline
The time line for the patent application would not start when the part is listed in the registry. Instead, would begin when the application of the part has been publicly disclosed. Patentability lies in the combinations of parts that together provide a function. Parts alone may not be patentable where they are not novel or where the innovation is to small to be considered non-obvious.
Moving Forward
Patentability of Bacto Blood may depend on what aspects of the invention are claimed in a patent application. The aspects of the invention could include:
1. Methods of using Bacto Blood
2. Composition of Bacto Blood
1. The system as a whole
2. Parts of the system or devices with in the E. coli chassis
3. Methods of making Bacto Blood
Each aspect be included as a claim or a set of claims in the application. Thus each aspect may be separately patentable.
Take home point
It is the sum of parts that become patentable when put together in an applicable system. Parts can still be listed in a public registry as means to disseminate knowledge. The parts may be well characterized. The parts may not be made for just one specific use in one specific system. The purpose of having well characterized and standardized parts is that these parts can be used in many different systems. Different systems may remain separately patentable despite haiving in common a single part among their many parts. In some situations, a part that has multiple applications may be separately patentable for each of its methods of use.
Further Questions
1. Can we provide distinguishable definition of a part so that we can distinguish between a device that is patentable and a part is not patentable?
a. Shows critical limitations of such expertise like patent lawyers.
2. Given the challenge of integrating an open source approach with current IP practices in Biotechnology, how might synthetic biology be a driver for inventing new modes industrial practices and partnerships?
3. How does one design research protocols that draw on both biological sciences and human practices?
**These three questions show the limitations to the technical answers provided above**