BerkiGEM2007 WikiPlaying2
From 2007.igem.org
| Line 43: | Line 43: | ||
} | } | ||
h2 { | h2 { | ||
| + | font-size: x-large; | ||
| + | color: #000000; | ||
| + | } | ||
| + | h3 { | ||
font-size: large; | font-size: large; | ||
color: #000000; | color: #000000; | ||
} | } | ||
| + | .style1 {font-size: x-small} | ||
--> | --> | ||
</style> | </style> | ||
| Line 54: | Line 59: | ||
<div align="center"><a href="https://2007.igem.org/Berkeley_UC"><<< Return to UC Berkeley iGEM 2007 </a></div> | <div align="center"><a href="https://2007.igem.org/Berkeley_UC"><<< Return to UC Berkeley iGEM 2007 </a></div> | ||
<p align="center"><a href="https://2007.igem.org/BerkiGEM2007Present4">Next >></a></p> | <p align="center"><a href="https://2007.igem.org/BerkiGEM2007Present4">Next >></a></p> | ||
| - | <h1 align="center">Engineering Bactoblood for Oxygen Transport< | + | <h1 align="center">Engineering Bactoblood for Oxygen Transport</h1> |
| - | </ | + | <h3 align="center">Introduction<br /> |
| - | + | </h3> | |
<p>The primary function of human erythrocytes is to transport oxygen to the body's tissues and remove CO2. This is accomplished principly by high concentrations of the protein hemoglobin. However, functional expression of hemoglobin requires the coexpression of the small molecule (heme) that specifically binds oxygen, proteins that promote the expression, folding, and addition of heme to hemoglobin, and proteins that maintain the oxidation state of hemoglobin and prevent the accumulation of toxic oxidizing species in the cell. Bactoblood will similarly require these activities, so we designed a hierarchical genetic device that encoded this oxygen transport function. Our design contains a heme biosynthesis devices, a hemoglobin generation device, a chaperone device, and a detoxifying device. Additionally, we investigated alternatives to hemoglobin that may provide superior oxygen transport to Bactoblood than their human counterpart.</p> | <p>The primary function of human erythrocytes is to transport oxygen to the body's tissues and remove CO2. This is accomplished principly by high concentrations of the protein hemoglobin. However, functional expression of hemoglobin requires the coexpression of the small molecule (heme) that specifically binds oxygen, proteins that promote the expression, folding, and addition of heme to hemoglobin, and proteins that maintain the oxidation state of hemoglobin and prevent the accumulation of toxic oxidizing species in the cell. Bactoblood will similarly require these activities, so we designed a hierarchical genetic device that encoded this oxygen transport function. Our design contains a heme biosynthesis devices, a hemoglobin generation device, a chaperone device, and a detoxifying device. Additionally, we investigated alternatives to hemoglobin that may provide superior oxygen transport to Bactoblood than their human counterpart.</p> | ||
| - | + | <p> </p> | |
| - | <p | + | |
| - | + | ||
| - | + | ||
<h2 align="center">The Hemoglobin Generating Device</h2> | <h2 align="center">The Hemoglobin Generating Device</h2> | ||
<p align="center"><img src="https://static.igem.org/mediawiki/2007/d/d1/Berk-Figure-HbA-HbB.png" alt="" name="HemoglobinCassette" width="337" height="111" id="HemoglobinCassette" /></p> | <p align="center"><img src="https://static.igem.org/mediawiki/2007/d/d1/Berk-Figure-HbA-HbB.png" alt="" name="HemoglobinCassette" width="337" height="111" id="HemoglobinCassette" /></p> | ||
| + | <h3 align="left">Background: </h3> | ||
<p>The primary component needed for efficient oxygen transport in our system is human hemoglobin A. (HbA) HbA is a tetramer that consists of two different subunits, α2β2. </p> | <p>The primary component needed for efficient oxygen transport in our system is human hemoglobin A. (HbA) HbA is a tetramer that consists of two different subunits, α2β2. </p> | ||
| - | < | + | <h3>Problems to Overcome: </h3> |
| - | + | <p>There are several problems which we have addressed with regards to hemoglobin and oxygen transport. The first of which is the insufficiently low P50, the partial pressure of oxygen needed for 50% saturation. A low P50 means that the oxygen affinity is too high, which will inhibit the ability of the hemoglobin to deliver oxygen to the needed tissues. The P50 for wild type human hemoglobin is ~3.8 at physiological conditions, however this varies with temperature and pH. We aim to have a P50 in the range of ~30-40 torr, however, it has been suggested that there may not be an upper limit on the value of the P50. </p> | |
| - | + | <h3>Natural Solution:</h3> | |
| - | + | <p><img src="https://static.igem.org/mediawiki/2007/e/e6/P50Comparison.gif" alt="" width="562" height="488" align="left">In human erythrocytes, the problem of oxygen binding affinity is fixed by the presence of an allosteric modifiers, primarily 2,3-diphosphoglycerate (2,3-DPG), which forces the hemoglobin conformation into the lower affinity deoxy state, or the T-state. By pushing the hemoglobin into the T-state, 2,3-DPG is effectively pushing any bound oxygen out of the heme center. This effectively lowers the oxygen binding affinity of the hemoglobin and increases the P50. An increase of 0.4mM in DPG concentration decreases oxygen affinity by about 1.0 torr. Since the normal concentration of DPG in erythrocytes is ~5mM, this raises the P50 to ~16.3 torr. <br> | |
| + | <br /> | ||
</p> | </p> | ||
<p>In our system, we have chosen to use well studied mutants of human hemoglobin which have been engineered to be permanently in the deoxy T-state. The mutations are named Presbyterian (beta-Asn108Lys) and providence (beta-Lys82Asp).<br /> | <p>In our system, we have chosen to use well studied mutants of human hemoglobin which have been engineered to be permanently in the deoxy T-state. The mutations are named Presbyterian (beta-Asn108Lys) and providence (beta-Lys82Asp).<br /> | ||
The P50 is also sensitive to various ions such as Cl-, although to a much smaller amount than 2,3-DPG. The portion of hemoglobin that binds these types of modifiers is at the N-terminal end. When hemoglobin is expressed in E. coli, there is an extra methionine residue which is normally cleaved off in eukaryotic cells.</p> | The P50 is also sensitive to various ions such as Cl-, although to a much smaller amount than 2,3-DPG. The portion of hemoglobin that binds these types of modifiers is at the N-terminal end. When hemoglobin is expressed in E. coli, there is an extra methionine residue which is normally cleaved off in eukaryotic cells.</p> | ||
| + | <p> </p> | ||
| + | <p> </p> | ||
| + | <p> </p> | ||
| + | <p> </p> | ||
| + | <p><span class="style1">Image taken from George J. Brewer's Review Article: "2,3-DPG and Erythrocyte Oxygen Affinity", 1974</span></p> | ||
| + | <p> </p> | ||
| + | <h2 align="center">Heme Biosynthesis Device</h2> | ||
| + | <p align="center"><img src="https://static.igem.org/mediawiki/2007/f/fd/Berk-Figure-hemABCD.png" alt="" width="563" height="122" /></p> | ||
| + | <p><br /> | ||
| + | Heme is a prosthetic group to hemoglobin. Generally, heme consists of an iron atom surrounded by a porphyrin ring. Each hemoglobin molecule is capable of binding up to four heme groups. One of the most important functions of heme is to assist in the transportation of diatomic gases. In red blood cells, heme and hemoglobin are the components that allow the binding of oxygen. As a result of this interaction, red blood cells continuously deliver oxygen to the entire body.</p> | ||
| + | <h2 align="center"> </h2> | ||
<p align="center"> </p> | <p align="center"> </p> | ||
<h2 align="center">The Chaperone Device</h2> | <h2 align="center">The Chaperone Device</h2> | ||
Revision as of 08:19, 26 October 2007
Engineering Bactoblood for Oxygen Transport
Introduction
The primary function of human erythrocytes is to transport oxygen to the body's tissues and remove CO2. This is accomplished principly by high concentrations of the protein hemoglobin. However, functional expression of hemoglobin requires the coexpression of the small molecule (heme) that specifically binds oxygen, proteins that promote the expression, folding, and addition of heme to hemoglobin, and proteins that maintain the oxidation state of hemoglobin and prevent the accumulation of toxic oxidizing species in the cell. Bactoblood will similarly require these activities, so we designed a hierarchical genetic device that encoded this oxygen transport function. Our design contains a heme biosynthesis devices, a hemoglobin generation device, a chaperone device, and a detoxifying device. Additionally, we investigated alternatives to hemoglobin that may provide superior oxygen transport to Bactoblood than their human counterpart.
The Hemoglobin Generating Device

Background:
The primary component needed for efficient oxygen transport in our system is human hemoglobin A. (HbA) HbA is a tetramer that consists of two different subunits, α2β2.
Problems to Overcome:
There are several problems which we have addressed with regards to hemoglobin and oxygen transport. The first of which is the insufficiently low P50, the partial pressure of oxygen needed for 50% saturation. A low P50 means that the oxygen affinity is too high, which will inhibit the ability of the hemoglobin to deliver oxygen to the needed tissues. The P50 for wild type human hemoglobin is ~3.8 at physiological conditions, however this varies with temperature and pH. We aim to have a P50 in the range of ~30-40 torr, however, it has been suggested that there may not be an upper limit on the value of the P50.
Natural Solution:
 In human erythrocytes, the problem of oxygen binding affinity is fixed by the presence of an allosteric modifiers, primarily 2,3-diphosphoglycerate (2,3-DPG), which forces the hemoglobin conformation into the lower affinity deoxy state, or the T-state. By pushing the hemoglobin into the T-state, 2,3-DPG is effectively pushing any bound oxygen out of the heme center. This effectively lowers the oxygen binding affinity of the hemoglobin and increases the P50. An increase of 0.4mM in DPG concentration decreases oxygen affinity by about 1.0 torr. Since the normal concentration of DPG in erythrocytes is ~5mM, this raises the P50 to ~16.3 torr.
In human erythrocytes, the problem of oxygen binding affinity is fixed by the presence of an allosteric modifiers, primarily 2,3-diphosphoglycerate (2,3-DPG), which forces the hemoglobin conformation into the lower affinity deoxy state, or the T-state. By pushing the hemoglobin into the T-state, 2,3-DPG is effectively pushing any bound oxygen out of the heme center. This effectively lowers the oxygen binding affinity of the hemoglobin and increases the P50. An increase of 0.4mM in DPG concentration decreases oxygen affinity by about 1.0 torr. Since the normal concentration of DPG in erythrocytes is ~5mM, this raises the P50 to ~16.3 torr.
In our system, we have chosen to use well studied mutants of human hemoglobin which have been engineered to be permanently in the deoxy T-state. The mutations are named Presbyterian (beta-Asn108Lys) and providence (beta-Lys82Asp).
The P50 is also sensitive to various ions such as Cl-, although to a much smaller amount than 2,3-DPG. The portion of hemoglobin that binds these types of modifiers is at the N-terminal end. When hemoglobin is expressed in E. coli, there is an extra methionine residue which is normally cleaved off in eukaryotic cells.
Image taken from George J. Brewer's Review Article: "2,3-DPG and Erythrocyte Oxygen Affinity", 1974
Heme Biosynthesis Device

Heme is a prosthetic group to hemoglobin. Generally, heme consists of an iron atom surrounded by a porphyrin ring. Each hemoglobin molecule is capable of binding up to four heme groups. One of the most important functions of heme is to assist in the transportation of diatomic gases. In red blood cells, heme and hemoglobin are the components that allow the binding of oxygen. As a result of this interaction, red blood cells continuously deliver oxygen to the entire body.
The Chaperone Device
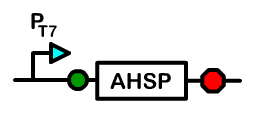
The alpha subunit is more prone to precipitation because an alpha-alpha dimer is insoluble under normal conditions. This can cause an excess of beta subunits and decreased functional hemoglobin output.
The solution in erythrocytes is to use a chaperone named alpha hemoglobin stabilizing protein (AHSP), which binds to single alpha subunits and facilitates the transfer of a beta subunit to the alpha. An alpha-beta dimer remains soluble. Our use of the bacterial chassis as a container that mimics an erythrocyte allows us to provide the same solution as in an erythrocyte. We include in our system the gene that encodes for human AHSP. In addition, we have also explored a fusion of di-alpha subunits with a glycine linker. This has been proven to give a higher soluble output by somehow stabilizing the alpha subunits and preventing precipitation.
Autoxidation Control


During the process of binding and unbinding oxygen, the four heme groups of the hemoglobin may spontaneously undergo autoxidation, ultimately causing the formation of damaging free radicals. This causes two problems. The first problem is that the free radicals can cause damage to cellular proteins, affecting their function. Another problem is that when autoxidation occurs, an electron is transferred from the Fe2+ iron center to the oxygen, creating a superoxide and leaving the heme with a Fe3+ metal center. The resulting hemoglobin is known as methemoglobin and it is unable to carry oxygen. The autoxidation occurs to a significant amount on the order of hours.
Erythrocytes remedy the problem of free radical accumulation and damage by containing the antioxidant enzymes catalase and superoxide dismutase. These enzymes catalyze the breakdown of superoxide into oxygen and H2O. Human erythrocytes have addressed the autoxidation problem by using the NADH dependent enzyme, cytochrome b5 reductase. The basic mechanism is shown here.
Hemoglobin Alternatives
We also investigated two alternatives to the hemoglobin part in our device: H-NOX and Myoglobin. Although the intrinsic oxygen-carrying ability of these proteins is different from Hemoglobin, variants of these proteins have been engineered with similar P50 values. These variants might allow Bactoblood to carry more oxygen than hemoglobin.
H-NOX is a heme-based sensor that is found in bacteria. H-NOX is able to bind Oxygen using a distal pocket tyrosine. For this gene I added the T7 promoter we created for this project, an RBS site, and lastly a Bca1092 terminator. When we assayed this part the results were inconclusive. The part was assembled correctly but the assay didn't show strong signs of expression.
The second gene we explored was Sperm Whale Myoglobin. Myoglobin is a monomeric protein that behaves as an intracellular oxygen storage site. Sperm whale myoglobin in particular is easily found in large amounts in the whale's muscle tissue. The construction of this part was very similar to that of the H-NOX composite part. It used the same promoter, terminator, and RBS. The assay for Myoglobin showed a bit more promise but couldn't conclusively show that Myoglobin was binding to oxygen.