CSHL
From 2007.igem.org
(→Genetics) |
|||
| Line 1: | Line 1: | ||
=Engineering fruit fly behavior by remote activation of neurons involved in reward and punishment= | =Engineering fruit fly behavior by remote activation of neurons involved in reward and punishment= | ||
| - | Keywords: behavior, remote control, engineering, | + | Keywords: behavior, remote control, engineering, ''Drosophila melanogaster'', Channelrhodopsin-2, blue light, UAS GAL4 system |
[[Image:Drosophila.jpg]] | [[Image:Drosophila.jpg]] | ||
| Line 43: | Line 43: | ||
===Anticipated Challenges=== | ===Anticipated Challenges=== | ||
| - | One major difficulty in achieving adult flies that behaviorally respond to blue light is the opacity of the | + | One major difficulty in achieving adult flies that behaviorally respond to blue light is the opacity of the ''Drosophila'' chitin cuticle. If the blue light cannot reach the neurons of the brain it will have no influence. So a major engineering hurdle is to create some sort of window in its skull that allows the blue light to penetrate down to the brain tissue. If this can't be accomplished we will work on training the ''Drosophila'' larvae, which have transparent cuticles and a very apparent reaction (contraction) to blue light in those larvae expressing ChR2 pan-neuronally. The limitations of using the larvae instead of the adults is that presumably the larvae have less capacity for learning. In addition, the larvae have no sight, so we can't use visual signals as conditioned stimuli. |
Another matter could be the stability of the all-trans retinal which is the cofactor required by Channelrhodopsin-2 to be functional. This cofactor is added in the larval diet and assimilated by the larvae which respond to blue-light, however during the metamorphosis to the adult form, many metabolic changes occur and the co-factor could be eliminated in the adult. | Another matter could be the stability of the all-trans retinal which is the cofactor required by Channelrhodopsin-2 to be functional. This cofactor is added in the larval diet and assimilated by the larvae which respond to blue-light, however during the metamorphosis to the adult form, many metabolic changes occur and the co-factor could be eliminated in the adult. | ||
| Line 68: | Line 68: | ||
====Larval Experiments==== | ====Larval Experiments==== | ||
| - | Our first experiments will involve the larvae of | + | Our first experiments will involve the larvae of ''Drosophila'' expressing ChR2. The following experiments are planned: |
| - | 1. In | + | 1. In ''Drosophila'' larvae expressing Channelrhodopsin-2 in all neurons (under either the elav or Mj85b promoter), when blue light is shone we expect to see contraction of the larval body, based on [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=16950113&ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum prior research] (ref. 2). In our experiments we have confirmed this response. We have taken videos of the larva expressing ChR2 pan-neuronally, with one or two copies of the ChR2 gene, under either the elav or Mj85b promoter. We plan to analyze these videos according to the following parameters: intensity of the light (based on the light's distance), speed of larvae prior to ChR2 activation, extent of contraction, recovery time (from immobility and disorientation) of the larvae after ChR2 activation, and speed of the larvae under continuous blue light (after recovery and accommodation to the blue light). |
2. We plan to test whether the larvae respond to and learn from punishing and rewarding stimuli by activating ChR2 expressed under the TH (dopaminergic, punishing) promoter and the dTD (octopaminergic, rewarding) promoter. We expect that that larvae normally show equal preference for leftward movement as for rightward movement, and this will be measured, in the larval locomotion on agar in a 5 cm 2D arena (petri dish). We will then measure the preference for rightward or leftward movement after the application of reward (or punishment) reinforcing a particular direction. If it can be shown that the larvae can learn a directional preference by the application of blue light, it will demonstrate that the dopaminergic/octopaminergic drivers are working properly and that their activation does in fact induce reward and punishment as expected. | 2. We plan to test whether the larvae respond to and learn from punishing and rewarding stimuli by activating ChR2 expressed under the TH (dopaminergic, punishing) promoter and the dTD (octopaminergic, rewarding) promoter. We expect that that larvae normally show equal preference for leftward movement as for rightward movement, and this will be measured, in the larval locomotion on agar in a 5 cm 2D arena (petri dish). We will then measure the preference for rightward or leftward movement after the application of reward (or punishment) reinforcing a particular direction. If it can be shown that the larvae can learn a directional preference by the application of blue light, it will demonstrate that the dopaminergic/octopaminergic drivers are working properly and that their activation does in fact induce reward and punishment as expected. | ||
| Line 100: | Line 100: | ||
5. Finally, if the first 4 adult experiments are accomplished we would like to see if the learned associations of the adult fruit-fly can also be applied to behavior in flight. We will use the FlightSimulator for these experiments. | 5. Finally, if the first 4 adult experiments are accomplished we would like to see if the learned associations of the adult fruit-fly can also be applied to behavior in flight. We will use the FlightSimulator for these experiments. | ||
| - | + | ====Experimental Apparatus==== | |
| - | + | =====CameraApparatus===== | |
| - | + | =====LarvaeSetupDish===== | |
| - | + | =====AdultArena1D===== | |
| - | + | =====AdultArena2D===== | |
| - | + | =====GluingStage===== | |
| - | + | =====FlightSimulator===== | |
<br /> | <br /> | ||
Revision as of 17:25, 1 July 2007
Contents |
Engineering fruit fly behavior by remote activation of neurons involved in reward and punishment
Keywords: behavior, remote control, engineering, Drosophila melanogaster, Channelrhodopsin-2, blue light, UAS GAL4 system
Team Members
Anh Nguyen
Martin Safrin
Laura Vibert
Liam Wang
Team Advisors
Partha Mitra
Josh Dubnau
Dan Valente
Hontao Qin
Project Description
Background and Motivations
The aim of this project is to engineer a behavior in the common fruit fly. It is well known that the fruit fly is capable of learning through reinforcement, and many experiments in classical and operant conditioning have been done to demonstrate the fly's capacity for learning and memory. By applying reward and punishment in the presence of certain neutral stimuli, the fly can make associations and learn to avoid or seek out these previously neutral stimuli. The current hypothesis in the literature is that, like humans, punishment and reward in insects are mediated by different neurotransmitters. It is believed that in insects, dopamine mediates punishment and octopamine (an invertebrate analog of norepinephrine) mediates reward. In our project we seek to further develop an existing method that allows for direct activation of these putative reward or punishment circuits by application of blue light to the intact animal. We hope to use this method to engineer defined, and even complex, behaviors in the fruit fly by using the blue light flashes to directly ‘reward’ or ‘punish’ behaving animals in real time.
A recently discovered membrane channel protein called [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=14615590&ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum Channelrhodopsin-2], derived from the alga Chlamydomonas, has been shown to be usable to remotely activate neurons by shining blue wavelength light (references [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=16950113&ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum 2], [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=16298005&ordinalpos=13&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum 3], [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17442243&ordinalpos=3&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum 4]). This channel protein is normally impermeable to ions, that is, it is normally in the closed state. But upon stimulation with blue light, the channel opens and allows permeation by positively charged ions. If this protein channel is transgenically expressed in a neuron, the activation of the channel by blue light causes the influx of positive ions, which in turn causes an action potential to be fired. Research confirms that these artificially induced action potentials can be controlled with great temporal precision by the application of blue light to the neuron or even to an intact brain. Furthermore, it has been shown that in fruit fly larvae, by localizing ChR2 to octopamine releasing neurons, blue light can be used replace naturally rewarding stimuli such as fructose, and by localizing ChR2 to dopamine releasing neurons, blue light can replace naturally punishing stimuli. In other words, the hypothesis that has been confirmed by in the literature is that activating octopaminergic neurons can substitute for a reward to the fly, and activating dopaminergic neurons acts as a punishment ([http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=14627633&ordinalpos=52&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum reference 5]). The advantage of using blue light over these naturally rewarding or punishing stimuli however is enormous because it can allow for quick and more temporally precise application of reward or punishment. Moreover, if we are able to use this approach to ‘shape’ the animals behavior, it will directly support the hypothesis that these neurotransmitters convey stimulus value to the animal.
There are two basic kinds of learning that can be applied in the fruit-fly. The first, classical conditioning, also known as Pavlovian conditioning, is the pairing of some neutral stimulus ('conditioned stimulus') with a stimulus that naturally elicits a certain kind of response ('unconditioned stimulus'). Under this procedure the natural response behavior is elicited by the new conditioned stimulus (CS). The classic example is of Pavlov ringing a bell at feeding time for his dogs. The feeding is the unconditioned stimulus that always, naturally, causes salivation. But when the bell is paired with just prior to feeding, pretty soon the bell on its own will come to stimulate a salivation response.
The second kind of conditioning, which we are employing, is called operant conditioning. Operant conditioning differs from classical conditioning in that it reinforces or punishes voluntary behavior. Reinforcement is simply something that increases the frequency of a particular behavior, while punishment decreases the frequency of the behavior. Punishment and reward can be positive or negative. For example, applying positive reinforcement is to give the favorable stimulus in response to a desired behavior. A negative reinforcement is the cessation of constant aversive stimulus, in response to a desired behavior. Positive punishment is the application of an aversive stimulus in response to an undesired behavior, and negative punishment is the removal of a favorable stimulus.
Our approach in engineering the behavior of the fruit-fly is to use operant conditioning as described above to shape their behavior. We will have two sets of populations of fruit-flies. In one, the blue light will serve as the favorable stimulus and in the other the blue light will serve as the aversive stimulus.
Experimental Approach
In our project ChR2 is localized to specific neural types by the use of the genetic tool known as the [http://biologie.univ-mrs.fr/upload/p100/GAL4_system_review.pdf UAS GAL4 system] (explained below). . This allows us to engineer fruit-flies that express ChR2 in only dopamine secreting cells, in only octopamine secreting cells, or pan-neuronally, to name a few possibilities.
Our goal is to use a training scheme that would associate behavioral actions of the animal with either octopamine or dopamine release. We will use the ChR2 expression in these neuron types to directly activate neuronal activity with blue light. Blue light flashes will be temporally paired with behavioral actions of the animals. If our hypothesis is correct, this will mimic reward or punishment depending upon whether we are expressing ChR2 in octopamine or dopamine neurons. In this case, we will shape the behavior of the animals.
For example, we may want to reward turning to the right in the presence of green light, and turning to the left in the presence of amber light. If this kind of context dependent learning works in the adult fly, we could potentially control the fly as if by remote control with these two lights.
Other potential directions for our engineering efforts include training the larvae instead of the adults, if engineering of the adults proves to be too difficult. Larvae learning behavior has already been described and particularly chemotaxis learning which allow the larva to associate a chemical stimulus in gradient with reward or punishment.
Anticipated Challenges
One major difficulty in achieving adult flies that behaviorally respond to blue light is the opacity of the Drosophila chitin cuticle. If the blue light cannot reach the neurons of the brain it will have no influence. So a major engineering hurdle is to create some sort of window in its skull that allows the blue light to penetrate down to the brain tissue. If this can't be accomplished we will work on training the Drosophila larvae, which have transparent cuticles and a very apparent reaction (contraction) to blue light in those larvae expressing ChR2 pan-neuronally. The limitations of using the larvae instead of the adults is that presumably the larvae have less capacity for learning. In addition, the larvae have no sight, so we can't use visual signals as conditioned stimuli.
Another matter could be the stability of the all-trans retinal which is the cofactor required by Channelrhodopsin-2 to be functional. This cofactor is added in the larval diet and assimilated by the larvae which respond to blue-light, however during the metamorphosis to the adult form, many metabolic changes occur and the co-factor could be eliminated in the adult.
Experimental Plan and Methods
Genetics
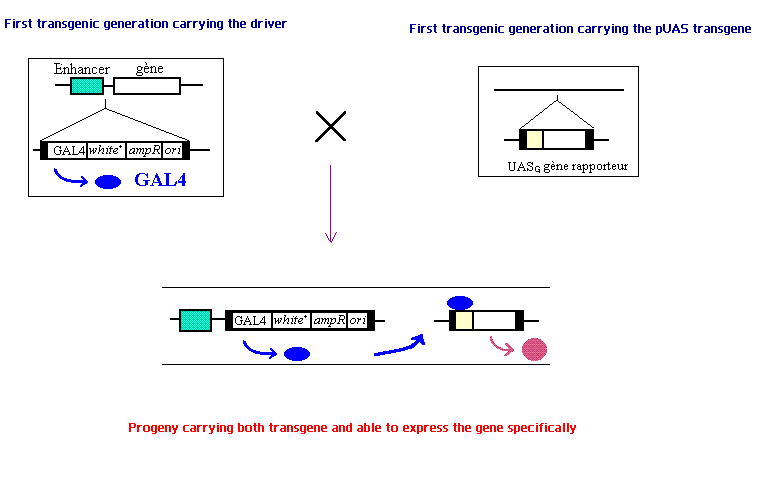
The genetic tool we use is the system UAS/GAL4. The system is a modular way of expressing a specified protein localized to a genetically defined population of cells. The system is composed of two transgenes. The first carries a gene encoding the GAL4 protein preceded by a promoter that is specific to only the type of cells where we want our desired protein to be expressed. The second transgene, called pUASg is composed of the UAS promoter sequence follow by the gene we want to express. This transgene, when inserted between a specific enhancer in the promoter region and a gene, allows the expression of the desired gene only in the cells we want to target. The UAS promoter sequence requires the GAL4 protein to start the translation of the sequence. In cells expressing GAL4 (as defined by the promoter regulating GAL4 expression) the GAL4 protein will bind the UAS promoter sequence of the pUASg transgene activating the translation and expression of the desired gene.
We will first use two pan-neuronal drivers, MJ8Bb and ElaV which allows the expression of the Channelrhodopsin-2 gene in all neurons. For that we cross two transgenic strains, one carrying the driver (Mj75b:GAL4, or ElaV:GAL4) and the other carrying UAS:Channelrhodopsin-2. These two drivers will be used for standardization of the obvious response (pan-neuronal activation) and to ensure the expression of the ChR2. For these crosses, the progeny are all heterozygous for both transgenes (the promoter:GAL4 unit, and the UAS:ChR2 unit).
For the behavior experiments, we will use the octopaminergic (dTD:Gal4) and dopaminergic (TH:GAL4) drivers.
Drosophila genetics is based on three main points: firstly there is no recombination in males. Secondly, some “modified” chromosomes called balancers can not recombine and individuals carrying them can be recognized phenotypically. Finally we know some markers and their positions.
For these reasons, Drosophila is a practical genetic model. We will use all this genetic possibilities to create two homozygous stable strains carrying the UAS:ChR2 construct, and in one case the dTD:GAL4 driver and in the other case, the TH:GAL4 driver.
The UAS/GAL4 system is a common genetic tool for tageting gene expression in Drosophila. A protein coded by GAL4, first identified in the yeast Saccharomyces cerevisiae, is a regulator of gene transcription. This protein binds an Upstream Activating Sequences (UAS) element, which is analogous to an enhancer in multicellular eukaryoptes. The UAS element is essential for transcriptional activation of GAL4 regulated gene. Hence, the system is a modular way of expressing a specified protein localized to a genetically defined population of cells. In our project, we cross two strains of flies. One carries a gene encoding the GAL4 protein preceded by a promoter that is specific to only the type of cells where our desired protein is made. The other one carries a transgene called pUASg, which is composed of the UAS promoter sequence follow by the gene we want to express. This transgene, when inserted between a specific enhancer in the promoter region and a gene, allows the expression of the desired gene only in the cells we want to target. In cells expressing GAL4 (as defined by the promoter regulating GAL4 expression) the GAL4 protein will bind the UAS promoter sequence of the pUASg transgene which in turn will activate the expression of the desired gene.
In our project, we cross two transgenic strains, one carrying a pan-neuronal driver which is either MJ85b:GAL4 or ElaV:GAL4, and the other carrying UAS:Channelrhodopsin-2. The progeny of these crosses that are heterozygous for both transgenes will express Channelrhodopsin-2 in all neurons. They are used for the standardisation of obvious response (i.e. contraction of larvae when blue light is turned on) and to ensure the expression of ChR2 in neurons.
For the behavior experiments, we use strains containing either octopaminergic (dTD:Gal4) and dopaminergic (TH:GAL4) drivers which allow expression of ChR2 only in octopaminergic and dopaminergic neurons, respectively. Drosophila genetics is based on three main points. Firstly, there is no recombination in males. Secondly, some “modified” chromosomes called balancers can not recombine and individuals carrying them can be recognized phenotypically. Finally, some markers and their positions are known. We will use all these genetic advantages to create two homozygous stable strains carrying the UAS:ChR2 construct and either the dTD:GAL4 driver or the TH:GAL4 driver.
Larval Experiments
Our first experiments will involve the larvae of Drosophila expressing ChR2. The following experiments are planned:
1. In Drosophila larvae expressing Channelrhodopsin-2 in all neurons (under either the elav or Mj85b promoter), when blue light is shone we expect to see contraction of the larval body, based on [http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=16950113&ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum prior research] (ref. 2). In our experiments we have confirmed this response. We have taken videos of the larva expressing ChR2 pan-neuronally, with one or two copies of the ChR2 gene, under either the elav or Mj85b promoter. We plan to analyze these videos according to the following parameters: intensity of the light (based on the light's distance), speed of larvae prior to ChR2 activation, extent of contraction, recovery time (from immobility and disorientation) of the larvae after ChR2 activation, and speed of the larvae under continuous blue light (after recovery and accommodation to the blue light).
2. We plan to test whether the larvae respond to and learn from punishing and rewarding stimuli by activating ChR2 expressed under the TH (dopaminergic, punishing) promoter and the dTD (octopaminergic, rewarding) promoter. We expect that that larvae normally show equal preference for leftward movement as for rightward movement, and this will be measured, in the larval locomotion on agar in a 5 cm 2D arena (petri dish). We will then measure the preference for rightward or leftward movement after the application of reward (or punishment) reinforcing a particular direction. If it can be shown that the larvae can learn a directional preference by the application of blue light, it will demonstrate that the dopaminergic/octopaminergic drivers are working properly and that their activation does in fact induce reward and punishment as expected.
3. We plan to reward or punish the flies based on location. In this experiment the plan is to reward or punish the _Drosophila_ larva based on whether it is on one side of the arena or the other side. If we can establish a place preference it demonstrates again that the TH-GAL4 and dTD-GAL4 drivers are working, and that the larvae have a capacity for remembering its location in 2 dimensions.
4. Another behavior we will try to reinforce in the _Drosophila_ larvae is the head-lift. The larvae have a tendency to occasionally lift their head and 'look' left and right and then choose a new direction to move in. In this experiment we plan to reward (or cease punishment) the larva whenever it performs this action.
Adult Experiments
As mentioned, it seems that the chitin cuticle protecting the _Drosophila_ brain may be too opaque to allow blue light to penetrate. To get the Channelrhodopsin-2 system working in transgenic adult flies, this part of the cuticle may need to be removed. Unfortunately a fruit-fly with its brain exposed does not live very long, so we also need to create a protective barrier that is transparent to the blue light. One possibility is to use transparent glue.
When this is achieved we plan to do the following set of experiments:
1. On adult Drosophila expressing Channelrhodopsin-2 in all neurons (using the elav or Mj85b promoter in the UAS/GAL4 system), blue light will be shone. We are not exactly sure what we will see, but we expect that activating all neurons indiscriminately will create either an epileptic seizure-type reaction, some twitching, proboscis extension, or at the very least some observable response. If this is shown it is the first step towards demonstrating that ChR2 is working in the adult.
2. Our next experiment is to see if we can establish a simple movement preference learning in the adult fly. In flies expressing ChR2 under control of the TH promoter (dopaminergic neurons) we can shine blue light on the fly whenever it moves to the left or straight, and only cease when it moves to the right. If we can show statistically that the fly is showing increased preference towards rightward movement it can be seen that the activation of dopamingeric cells with Channelrhodopsin-2 is an effective means of punishment. Additionally, a similar experiment can be done by testing flies expressing ChR2 under control of the dTD promoter (octopaminergic neurons). If we shine blue light only when the fly moves to the right, and we see increased rightward movement we see that activating ChR2 in octopaminergic cells acts as an effective rewarding stimulus. These experiments can also be done to generate a leftward preference.
3. Another experiment will be performed that is similar to the previous one, but in this experiment we will try to establish a place preference instead of a directional preference. We will try two place preference assays. The 1D arena assay involves placing an adult fly on a track with one degree of freedom. In the center of the track will be the ChR2-activating (blue wavelength) light. We will measure if the ChR2-expressing flies show a location preference dependence based on the blue light that differs from other (non-ChR2 activating) wavelengths of light, and that also differs from the wild-type flies exposed to the blue wavelength. Additionally we will attempt to operantly condition the fly to prefer one side of a 2 dimensional arena.
4. Another experiment planned is to test whether the adult _Drosophila_ fly can learn to exhibit different behaviors based on context. The experiment will be administered as follows:
In the presence of constant green light we will reward flies expressing ChR2 under the dTD promoter (by shining blue light) whenever it moves to the right. Thus we establish that in the context of green light, rightward movement is rewarding. Then under constant amber-colored light, the fly will be rewarded when it moves to the left. Under this scheme it is hoped that the adult flies will learn to move rightward when green light is shone (along with the blue light reward, or cessation of blue light punishment), and to move leftward when amber-colored light is shone (again, along with blue light).
If two contexts can be learned and remembered by flies then an essential step in achieving a remote controllable Drosophila fly has been accomplished.
The activation of light and observation of behavior in both the larvae and the adults will be done manually at first, but may eventually be automated by video acquisition and tracking software.
5. Finally, if the first 4 adult experiments are accomplished we would like to see if the learned associations of the adult fruit-fly can also be applied to behavior in flight. We will use the FlightSimulator for these experiments.
Experimental Apparatus
CameraApparatus
LarvaeSetupDish
AdultArena1D
AdultArena2D
GluingStage
FlightSimulator