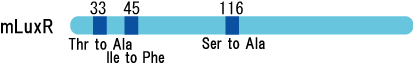Chiba/Quorum Sensing
From 2007.igem.org
|
Introduction | Project Design ( 1.Sticky Hands | 2.Communication | 3.Size Control ) | Making Marimos | Our Goal || Team Members | メンバ連絡簿 |
Size Control
Our Aim
Since produced AHL diffuses all around the bacteria culture, all receivers can ultimately aggregate to one huge marimo with their sticky hands. This final state is not the case of real marimos. Thus our idea for controlling the size of Bacteria Marimos is based on the high performance quorum sensing or AHL-diffusing inhibition.
- Raise the AHL productivity of Sender
- Increase the AHL sensitivity of Receiver
- Use the AHL degrading enzyme aiiA to localize AHL
1.Improving Sender
Design
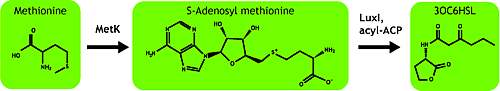
AHL is synthesized from methionine by the enzyme MetK and LuxI.
We tested in the hope of combined overexpression of these 2 genes will enhance the sender capacity.
Experiment
Sender
Ptet-LuxI
- Synthesize AHL constantly
metK Sender
- Synthesize AHL and express metK constantly
Receiver
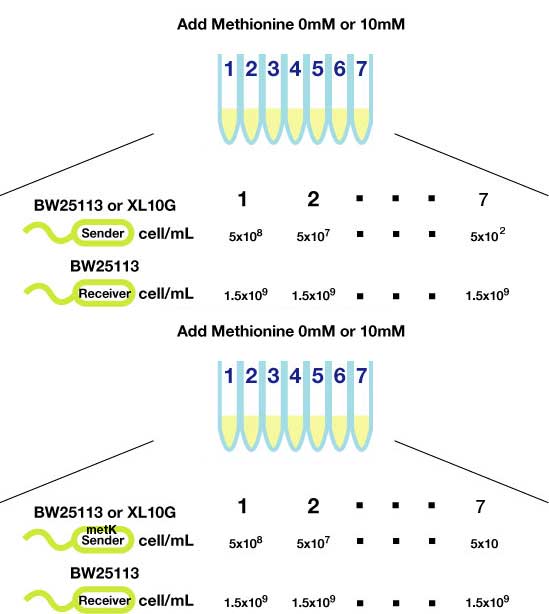
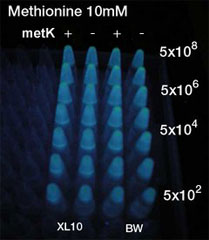
Method(Fig3.)
- Inoculated sender, MetK sender, and receiver in each liquid medias. Incubated at 37℃ 12h.
- Checked OD. Dispensed receiver in each tube equally. Spin downed senders and resuspended with fresh liquid media.
- Diluted senders to adjust cell population (5x108,5x107,......, 5x102).
- Mix receivers and senders.
- Incubated for 1h to 3h at room tempature
- Spindowned and UV checked.
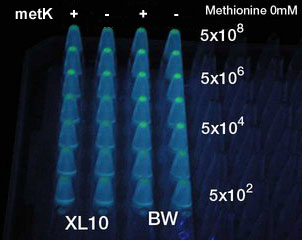
Result
- No difference was seen.
Between Methionine added or not. Between metK is expressed or not.
- 何と何の間の違いが見られないのかな?byとよたろ
Discussion
- No efficiency was observed.
- Our Assumption:S-adenosyl methionine was synthesized enough or consumed in other path way.
- Sequencing riquired.
2.Improving Receiver
Design
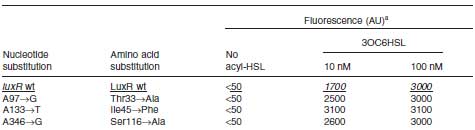
Collins et.al. described the hyper-sensitive variants of luxR to AHL.(Collins, C. H., Arnold, F. H. & Leadbetter, J. R. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005))
We created two types of sensitive luxR mutants.
Because there is no data shows 2point(I45F,S116A) mutated luxR.We expected something new data of mutation will be found. Also we want to obtain sensitive(not hyper sensitive) receiver.
Experiment
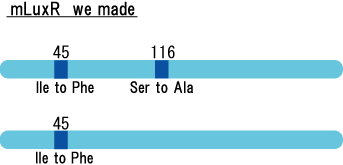
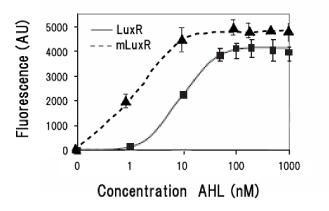
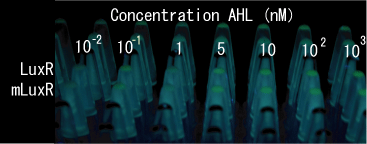
Sensitive luxR mutants assessed by follow method.
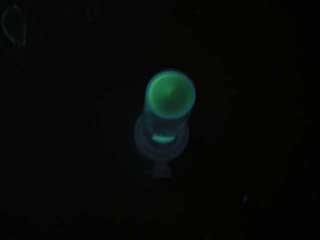
1.Inoculate Receiver(wild type luxR/BW25113), mutated Receiver(1point mutation/BW25113) in liquid media 37℃ 12hour. 2.Despence them equally. 3.Add AHL final concentration(1μM,100nM,10nM,5nM,1nM,0.1nM) 4.1hour at room temperature 5.Spindown and UV checked.
Result
- Both wild type and 1point mutation luxR express GFP under 5nM AHL concentration.
- 2point mutation(I45F,S116A) luxR Receiver express GFP without AHL.
Discussion
This table is quoted from paper.In the paper, used DH5α strain.
But wild type luxR expressed GFP under 5nM GFP concentration.
Assumption:There is some difference in strains.
3.Localizing AHL
Design1
![]()
AiiA expression is regulated by pLac, which makes it express constantly to inactivate AHL.
Experiment
- Inoculate E.coli carries this aiiA receiver plasmid in Liquid Media.
- At the same time, inoculate AHL sender E.coli in Liquid Media.
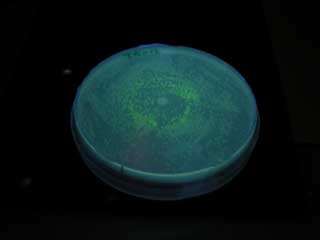
- Dilute receiver cells and spread on agar plate, and spot sender 1μL. Incubate at 37℃ 12hour
- Check plate.(GFP expressed?)
Result
GFP expression was not detected on the plate of aiiA receiver.
Discussion
Excessive amounts of aiiA is expressed to generate concentration gradient. We expected aiiA express more moderate to make it.
Design2
![]()
This gene circuit is expected so that aiiA is synthesized only when the receiver senses AHL signal. If so, the whole Bacteria Marimo becomes AHL quencher. In fact, the bacteria inside of the Marimo degrades AHL and the AHL diffusion outside of Marimo is inhibited.
Experiment
- Inoculate E.coli carries this aiiA receiver plasmid in Liquid Media.
- At the same time, inoculate AHL sender E.coli in Liquid Media.
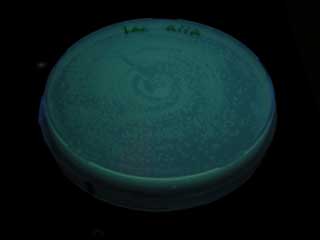
- Dilute receiver cells and spread on agar plate, and spot sender 1μL. Incubate at 37℃ 12hour
- Put plate 4℃
- Check GFP expression every 1hour.
- 実験方法は?by とよたろ
Result
- When we compared the GFP expression with the test tube of BBa_T9002, the bacteria with the consructed gene circuit did not fluoresce immediately after incubation, however fluoresced in 18~20 hour.
- しばらくすると、ってどのくらいのタイムスケールですか?by とよたろ
Discussion
- GFP expression means plux is activated. So we could make moderate aiiA receiver.
- But we need more good scheme.
Design3
![]()
Above picture describes aiiA is regulated inverted lux promoter with CI inverter.
- High AHL concentration: no aiiA expression, no AHL degrade.
- Low AHL concentration: aiiA expressed, AHL degraded.
図のようにインバーターをかませた.高い濃度ではaiiAが発現しないためAHLは分解されない.AHLが低い濃度ではaiiAが発現しAHLを分解する.
よってマリモの中心付近ではAHLを分解せず,外側へ行くと分解される.
Experiment
We could not finish assembling this part. It will be our future work. このパーツを作ろうとしたが、inverterを組み合わせた時点で終わってしまった。
Subpart:BBa_S03840