Cloning in BioBrick vectors
From 2007.igem.org
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | We | + | We amplified with [[PCR]] promoters and Pho80 coding sequence and we loaded 1µl on the agarose gel to see if there were amplification products,aspecific products or others.Then we digested our PCR products with XbaI and SpeI. |
[[1ORE-CYCtata]] | [[1ORE-CYCtata]] | ||
| Line 8: | Line 8: | ||
| - | We | + | We digested in two differents ways our plasmid to be sure of its identity! |
| - | Then we | + | Then we digested [[pSB1A3]][https://2007.igem.org/Biobrick_Vector_choice] with the same enzyme used for the insert. |
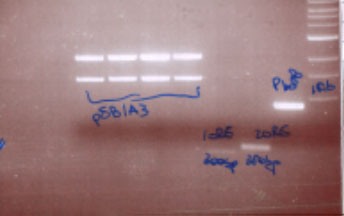
| - | We | + | We chose a plasmid containing one insert of 1,5kb so via gel extraction, we were able to separate the linearized plasmid from the insert. |
[[Image:pBs1A3.jpg]] | [[Image:pBs1A3.jpg]] | ||
| - | + | We performed ligations between pSB1A3 and 1ore,2ore and fox3. | |
| - | After ligations we | + | After ligations, we transformed plasmid pSB1A3 in competent cells and after mini&maxi-inoculations and MIDI-prep we loaded 1µl on the agarose gel. |
| Line 21: | Line 21: | ||
[[PHO80cds]] | [[PHO80cds]] | ||
| - | We decided to clone Pho80 coding sequence in pSB1A3 plasmid but since | + | We decided to clone Pho80 coding sequence in pSB1A3 plasmid but since its CDS has two XbaI restriction sites in positions |
| - | 91 and 648 | + | 91 and 648 we mutated these sites. We then cloned this gene in pSB1A3 plasmid using XbaI and SpeI enzymes for [[digestion]] of Pho80 mutated and pSB1A3 plasmid. |
| - | After this | + | After this ligation, we used EcoRI and SpeI enzymes to digest pSB1A3-Pho80 cassette and to clone it upstream of Rfp in pBca1020-r0040 vector. |
Latest revision as of 07:44, 26 October 2007
We amplified with PCR promoters and Pho80 coding sequence and we loaded 1µl on the agarose gel to see if there were amplification products,aspecific products or others.Then we digested our PCR products with XbaI and SpeI.
We digested in two differents ways our plasmid to be sure of its identity!
Then we digested pSB1A3[1] with the same enzyme used for the insert.
We chose a plasmid containing one insert of 1,5kb so via gel extraction, we were able to separate the linearized plasmid from the insert.
We performed ligations between pSB1A3 and 1ore,2ore and fox3. After ligations, we transformed plasmid pSB1A3 in competent cells and after mini&maxi-inoculations and MIDI-prep we loaded 1µl on the agarose gel.
We decided to clone Pho80 coding sequence in pSB1A3 plasmid but since its CDS has two XbaI restriction sites in positions 91 and 648 we mutated these sites. We then cloned this gene in pSB1A3 plasmid using XbaI and SpeI enzymes for digestion of Pho80 mutated and pSB1A3 plasmid. After this ligation, we used EcoRI and SpeI enzymes to digest pSB1A3-Pho80 cassette and to clone it upstream of Rfp in pBca1020-r0040 vector.