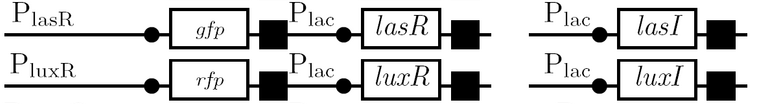Construction and Testing
From 2007.igem.org
(→Submitted Parts to the Registry) |
(→Construction Tree) |
||
| Line 9: | Line 9: | ||
*Parts and developing constructs all have abbreviated names for ease of labeling. To make the subtle distinction between a coding sequence and a coding sequence with a ribosome binding site, we use a ' on the computer and an underline when writing. A double ' or double underline indicates a coding sequence with a ribosome binding site and a transcriptional terminator. | *Parts and developing constructs all have abbreviated names for ease of labeling. To make the subtle distinction between a coding sequence and a coding sequence with a ribosome binding site, we use a ' on the computer and an underline when writing. A double ' or double underline indicates a coding sequence with a ribosome binding site and a transcriptional terminator. | ||
*Arrows show the direction of DNA cloning and specify which restriction enzymes to use for cutting. Hollow arrows are present when two parts are ligated from vectors of the same resistance, requiring additional methods such as the use of alkaline phosphatase, gel extraction, or a three-way ligation into a vector with different resistance. | *Arrows show the direction of DNA cloning and specify which restriction enzymes to use for cutting. Hollow arrows are present when two parts are ligated from vectors of the same resistance, requiring additional methods such as the use of alkaline phosphatase, gel extraction, or a three-way ligation into a vector with different resistance. | ||
| - | *The numbers 1-2-3-3.5-4 indicate which major protocols need to be carried at each step in the assembly cycle. | + | *The numbers 1-2-3-3.5-4 indicate which major protocols need to be carried out at each step in the assembly cycle: |
| + | 1) Transform selected DNA. | ||
| + | |||
| + | 2) Streak colonies from transformation spread plates. Patch on to plates of the opposite resistance. Innoculate culture tubes. | ||
| + | |||
| + | 3) Use the patches to discard colonies which are incorrect. Miniprep from the culture tubes of potentially correct colonies. Digest DNA with NotI and run on a gel. Discard colonies which have incorrect sizes. Innoculate tubes from correct colonies. | ||
| + | |||
| + | 3.5) Perform a full digest using appropriate enzymes on correct parts. Ligate parts together. | ||
| + | |||
| + | 4) Create a glycerol stock from the innoculation. | ||
<br style="clear:both;"/> | <br style="clear:both;"/> | ||
Revision as of 03:38, 27 October 2007
Contents |
Construction Tree
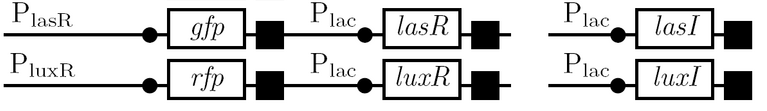
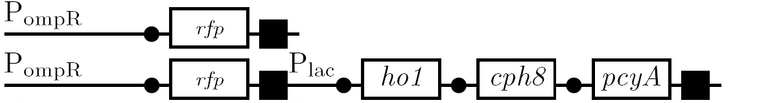
The diagram below outlines our parallel construction plan, from individual registry parts to the final half-adder. Because many people were working on the project at once, it is designed to keep everyone organised.
Diagram details:
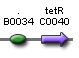
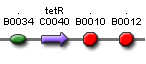
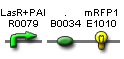
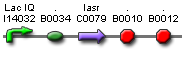
- Expected fragment sizes are shown at every node so gel results can be analyzed quickly.
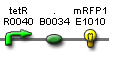
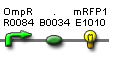
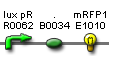
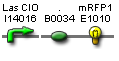
- Vector resistances for each part/construct are shown, with blue representing ampicillin resistance and red representing kanamycin resistance. Purple represents a resistance to both.
- Parts and developing constructs all have abbreviated names for ease of labeling. To make the subtle distinction between a coding sequence and a coding sequence with a ribosome binding site, we use a ' on the computer and an underline when writing. A double ' or double underline indicates a coding sequence with a ribosome binding site and a transcriptional terminator.
- Arrows show the direction of DNA cloning and specify which restriction enzymes to use for cutting. Hollow arrows are present when two parts are ligated from vectors of the same resistance, requiring additional methods such as the use of alkaline phosphatase, gel extraction, or a three-way ligation into a vector with different resistance.
- The numbers 1-2-3-3.5-4 indicate which major protocols need to be carried out at each step in the assembly cycle:
1) Transform selected DNA.
2) Streak colonies from transformation spread plates. Patch on to plates of the opposite resistance. Innoculate culture tubes.
3) Use the patches to discard colonies which are incorrect. Miniprep from the culture tubes of potentially correct colonies. Digest DNA with NotI and run on a gel. Discard colonies which have incorrect sizes. Innoculate tubes from correct colonies.
3.5) Perform a full digest using appropriate enzymes on correct parts. Ligate parts together.
4) Create a glycerol stock from the innoculation.
Testing Constructs
We use the fluorescent proteins GFP and RFP as reporter genes to test the functionality of some of the components and constructs of our half-adder design. Where possible, we make use of the lac inducible promoter to control expression of the enhancing or repressing elements.
Comparing basal and induced expression levels for the quorum sensing promoters
Testing functionality of the various promoters
Parts Submitted to the Registry
The following is a table listing all of the parts submitted to the registry.
Notes:
- ' denotes that the construct contains the ribosome binding site (RBS) and the coding region for that gene.
- ' ' denotes that the construct contains the ribosome binding site, coding region for that gene, and a transcriptional terminator.
Home | Project | Mathematical Modelling | Construction and Testing | Future Work