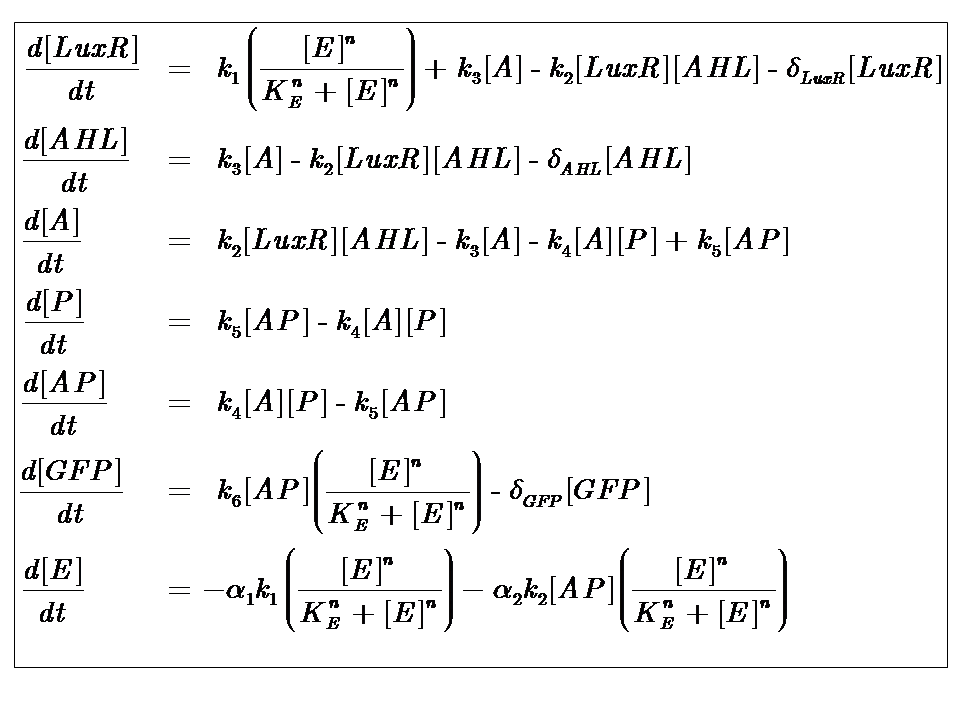Imperial/Infector Detector/Modelling
From 2007.igem.org
(→Abstract) |
(→''The Reaction Network'') |
||
| Line 36: | Line 36: | ||
====''The Reaction Network''==== | ====''The Reaction Network''==== | ||
| - | + | Both designs are based on the following reaction network: | |
| - | + | ||
| - | + | ||
| + | *AHL is assumed to diffuse freely "into" the system (we are dealing with a cell-free system, which comes into direct contact with the biofilm). | ||
| + | *The target AHL molecule binds with the monomeric protein LuxR. | ||
| + | *LuxR is either constitutively produced by construct 1, or directly introduced in purified form, as part of construct 2. | ||
| + | *The binding of these two proteins yields the intermediating LuxR-AHL complex, A. We call k<sub>2</sub> and k<sub>3</sub> the kinetic constants of the forward and backward reactions respectively. | ||
| + | *The formed transcription factor activates the transcription of the pLux operon, which codes for the relevant reporter protein, GFP. Activation occurs by way of the reversible binding of this transcription factor, A, to the response sequences in the operon (k<sub>4</sub> and k<sub>5</sub>) | ||
| + | *This leads to recruitment of RNA polymerase and increases the frequency of transcription initiation (Fuqua et al., 2001) of the construct gfp gene (strictly forward reaction, governed by k<sub>6</sub>). | ||
[[Image: IC07 Const1.png|thumb|left|440px|'''Figure 3''': Reaction network for Construct 1 (Energy-dependent)]] | [[Image: IC07 Const1.png|thumb|left|440px|'''Figure 3''': Reaction network for Construct 1 (Energy-dependent)]] | ||
[[Image: IC07 C2.png|thumb|right|440px|'''Figure 4''': Reaction network for Construct 2 (Energy-dependent)]] | [[Image: IC07 C2.png|thumb|right|440px|'''Figure 4''': Reaction network for Construct 2 (Energy-dependent)]] | ||
Revision as of 21:27, 26 October 2007

Infector Detector: Modelling
Introduction
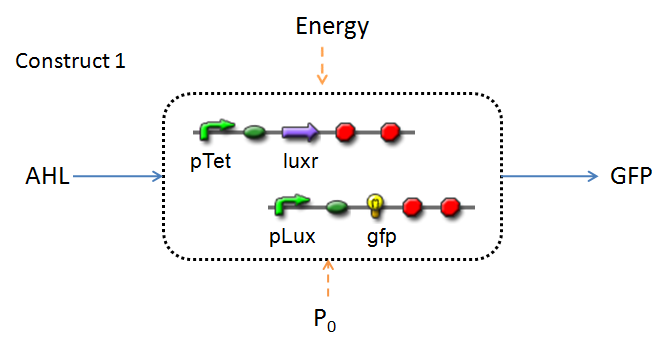
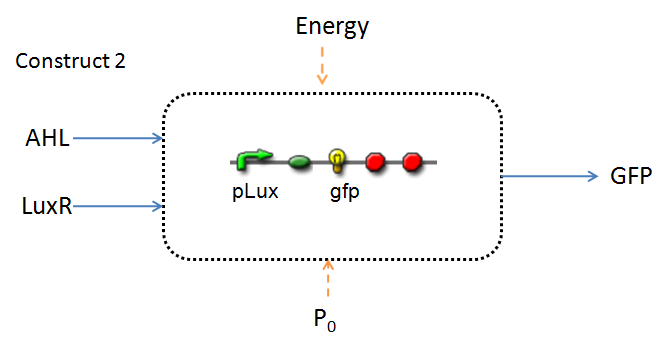
Infector Detector (ID) is a simple biological detector designed to expose the presence of a bacterial biofilm. It functions by exploiting the inherent AHL (Acetyl Homoserine Lactone) production employed by certain types of quorum-sensing bacteria, in the formation of such structures. The design phase of our project has yielded two possible system constructs.
Implementation & Reaction Network
In line with the concept of abstraction in Synthetic Biology, the correlation of the output of the proposed system constructs to their inputs, can be visualized by the following black-box illustrations of the two cases.
It is evident that AHL is an input to both constructs; a function of the particular biofilm. Furthermore, energy and promoter concentration are included as auxillary inputs to both system constructs. LuxR, is an additional input, exclusive to construct 2, which lacks constitutive expression of LuxR by pTET.
(this of course occuring within our cell-free chassis)
The Reaction Network
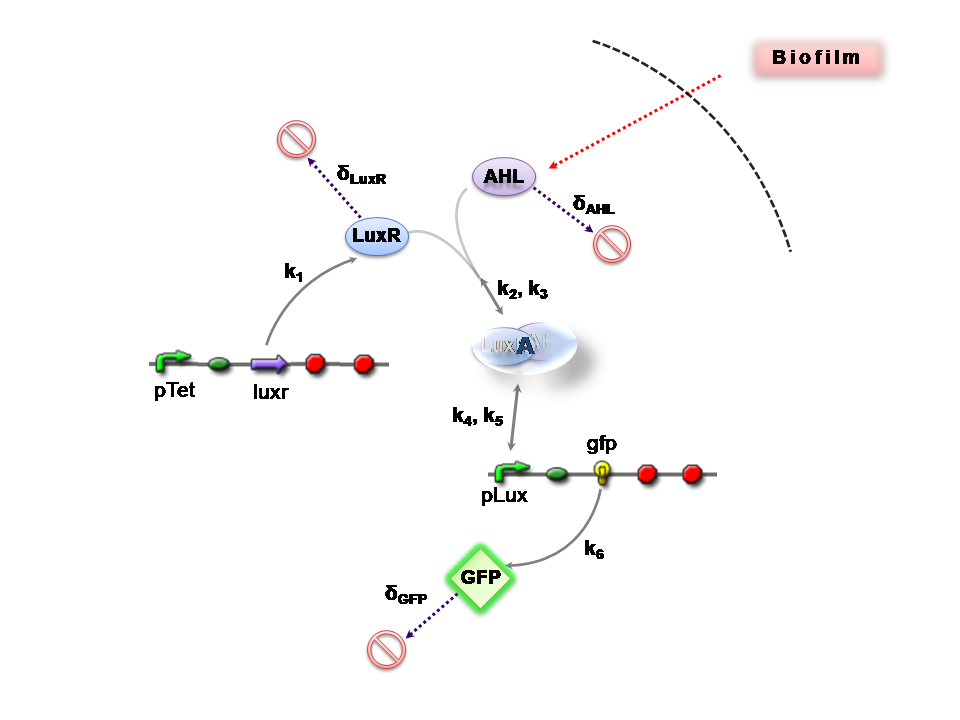
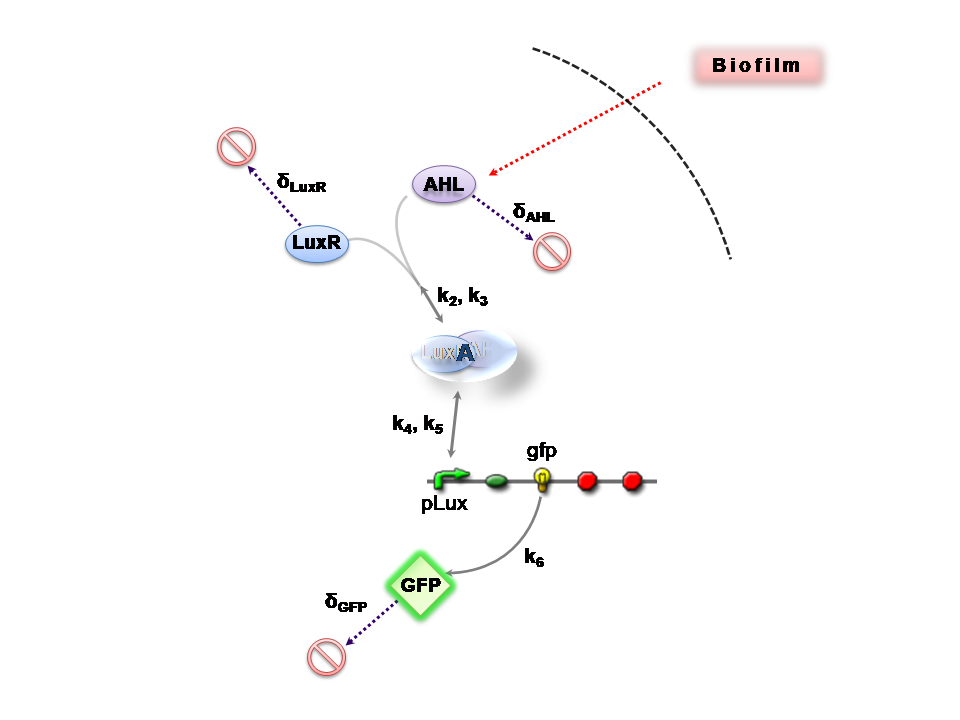
Both designs are based on the following reaction network:
- AHL is assumed to diffuse freely "into" the system (we are dealing with a cell-free system, which comes into direct contact with the biofilm).
- The target AHL molecule binds with the monomeric protein LuxR.
- LuxR is either constitutively produced by construct 1, or directly introduced in purified form, as part of construct 2.
- The binding of these two proteins yields the intermediating LuxR-AHL complex, A. We call k2 and k3 the kinetic constants of the forward and backward reactions respectively.
- The formed transcription factor activates the transcription of the pLux operon, which codes for the relevant reporter protein, GFP. Activation occurs by way of the reversible binding of this transcription factor, A, to the response sequences in the operon (k4 and k5)
- This leads to recruitment of RNA polymerase and increases the frequency of transcription initiation (Fuqua et al., 2001) of the construct gfp gene (strictly forward reaction, governed by k6).
Representative Model
In developing this model, we were interested in the behaviour at steady-state, that is when the system has equilibrated and the concentrations of the state variables remain constant.
At reasonably high molecular concentrations of the state variables, a continuous model can be adopted, which is represented by a system of ordinary differential equations.
It is for this reason that our approach to modelling the system follows a deterministic, continuous approximation.
We can condition the system in various manners, but for the purposes of our project, we will seek a formulation which is valid for both constructs considered, i.e. the governing equations are a represenation of both constructs.
The only difference is with regards to the parameter k1, the maximum transcription rate of the constitutive promoter (pTET). Therefore in construct 1, k1 is non-zero; k1 = 0 for construct 2 (which lacks pTET).
Our analysis took us through a number of models, but presented here is the most pertinent, most representative version. This model is based on energy-dependence (limited nutrient supply), which follows Hill-like dynamics.
The system kinetics are determined by the following coupled-ODEs. For a derivation of the governing equations, please access
Model Parameters
| Parameter | Description |
|---|---|
| Kinetic Constants | |
| k1 | Maximal constitutive transcription of LuxR by pTET |
| k2 | Binding between LuxR and AHL |
| k3 | Dissociation of protein complex LuxR-AHL (A) |
| k4 | Binding between A and pLux promoter |
| k5 | Dissociaton of A-pLux complex |
| k6 | Transcription of FP |
| Degradation Rates | |
| δLuxR | Degradation rate of LuxR |
| δAHL | Degradation rate of AHL |
| δGFP | Degradation rate of GFP |
| Hill Co-operativity | |
| n | Co-operativity coefficient describing the degree of energy dependence, which follows Hill-like dynamics |
| Energy consumption of transcription | |
| α1 | Energy consumption due to constitutive transcription of LuxR |
| α2 | Energy consumption due to transcription of gfp gene |
We now present the essential features of the system behaviour, simulated for a given set of parameters.
Simulations
Presented below are the most essential results of the simulations performed.
In light of the rapid equilibrium approximation which was employed in developing the model, relatively high k-values were selected:
k2 = k3 = k4 = k5 = 100 (dynamical equilibrium); k1 = 0.3 (constitutive transcription by pTet); k6 = 20*k1 (transcription of gfp);
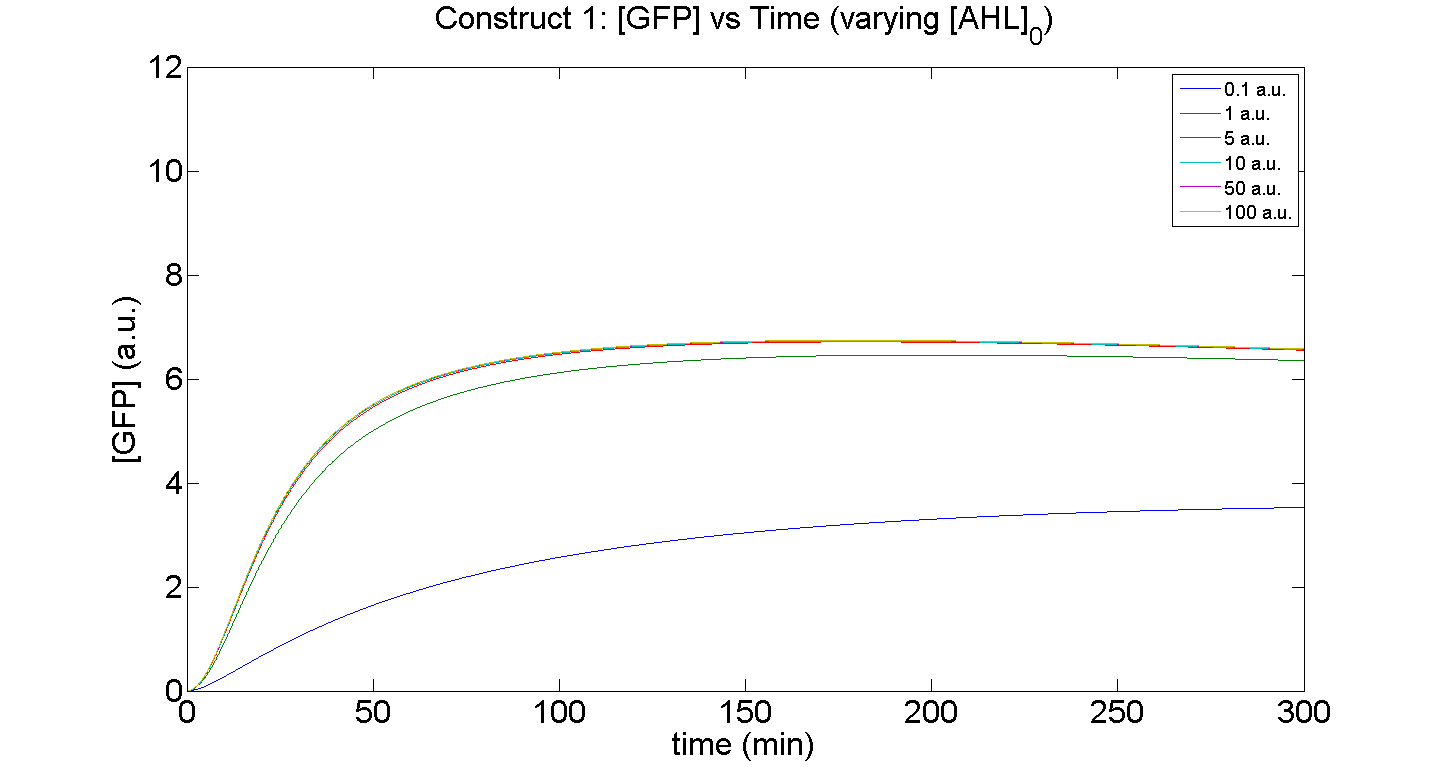
1. Construct 1: [GFP] vs time - varying [AHL]0
Discussion
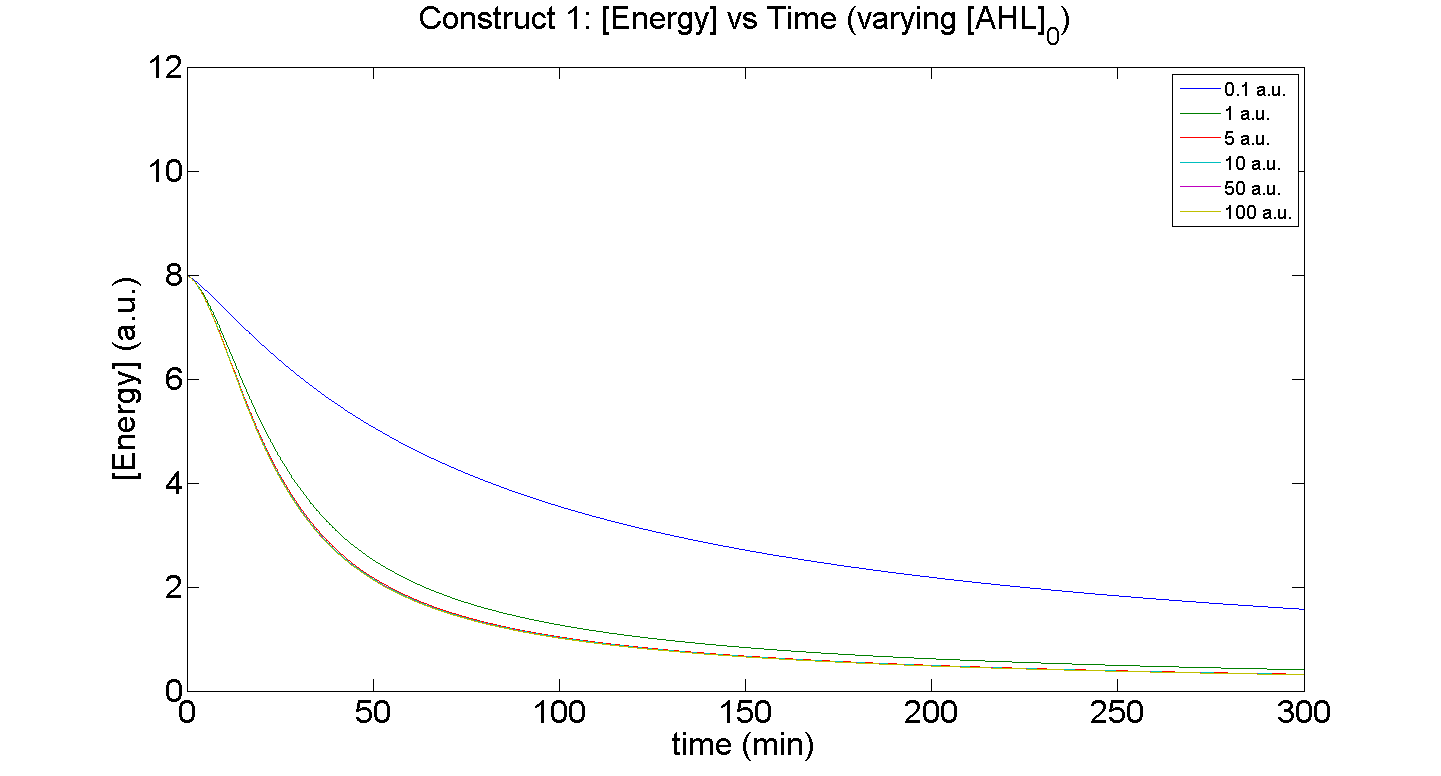
Figures 5 and 6 illustrate GFP expression and Energy depletion of construct 1, at various initial AHL concentrations.
The absolute value of the peak expression is a function of the various rate constants. Here our analysis serves to illustrate the general behaviour offered by construct 1 – a qualitative approach.
As initial [AHL] is increased, the level of expression increases accordingly to a point were there is negligible difference between the maximal outputs between adjacent tested cases of [AHL]. In fact, from this figure, and for this set of parameters, it is suggestive that saturation occurs at approximately 5 a.u.; in fact, the difference in maximal GFP output between when AHL is increased a several-fold is less than 10%.
Figure 6, the energy depletion plot, serves to illustrate the effect of increased initial concentrations of AHL on consumption of energy. More resources (promoters) need to be employed to "accommodate" the increasing [AHL]. This obviously increases the rate of energy depletion, until it levels off, as saturating behaviour has been attained - promoter saturation. This is the likely explanation for the "saturation curve" obtained from the experimental data, since the protein degradation terms themselves are almost negligible.
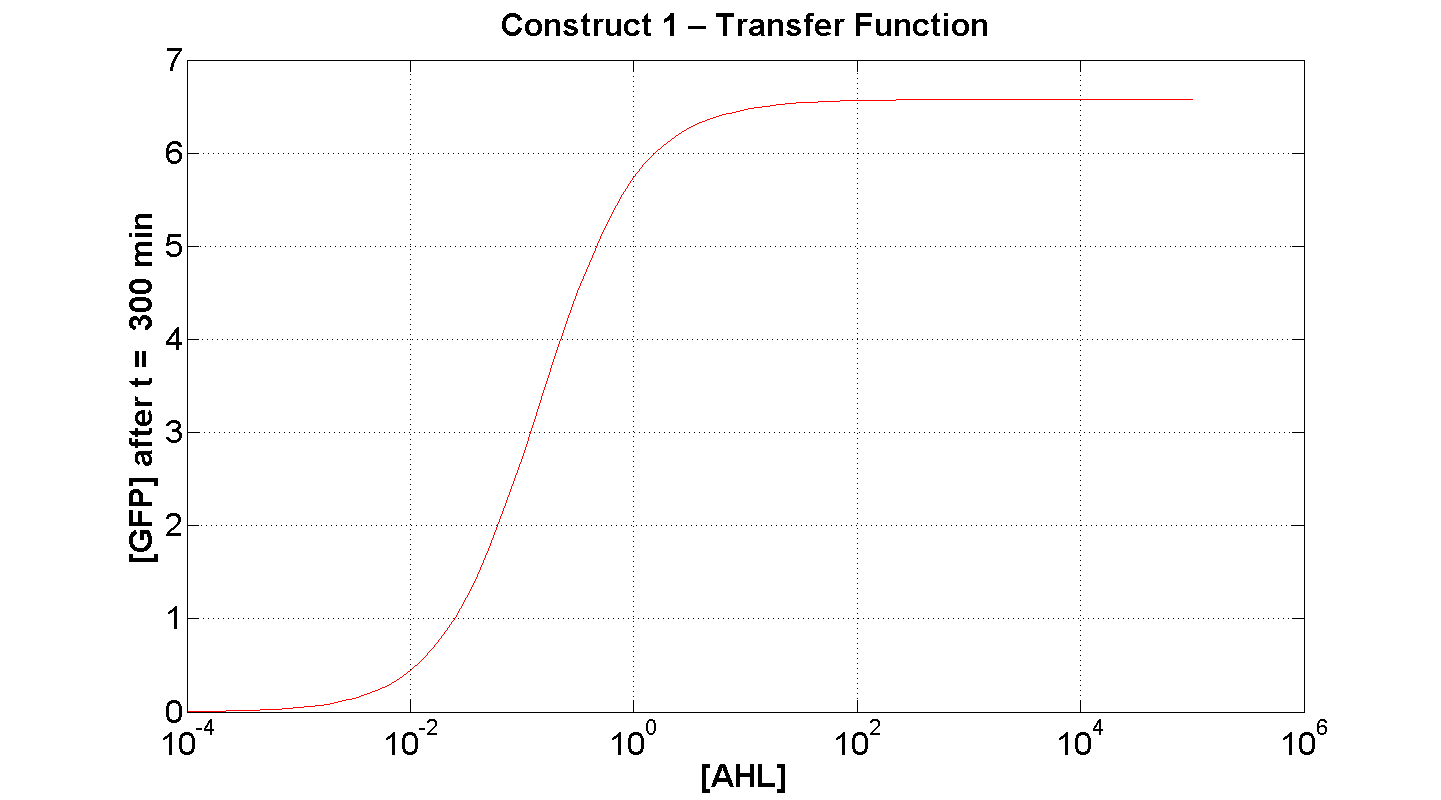
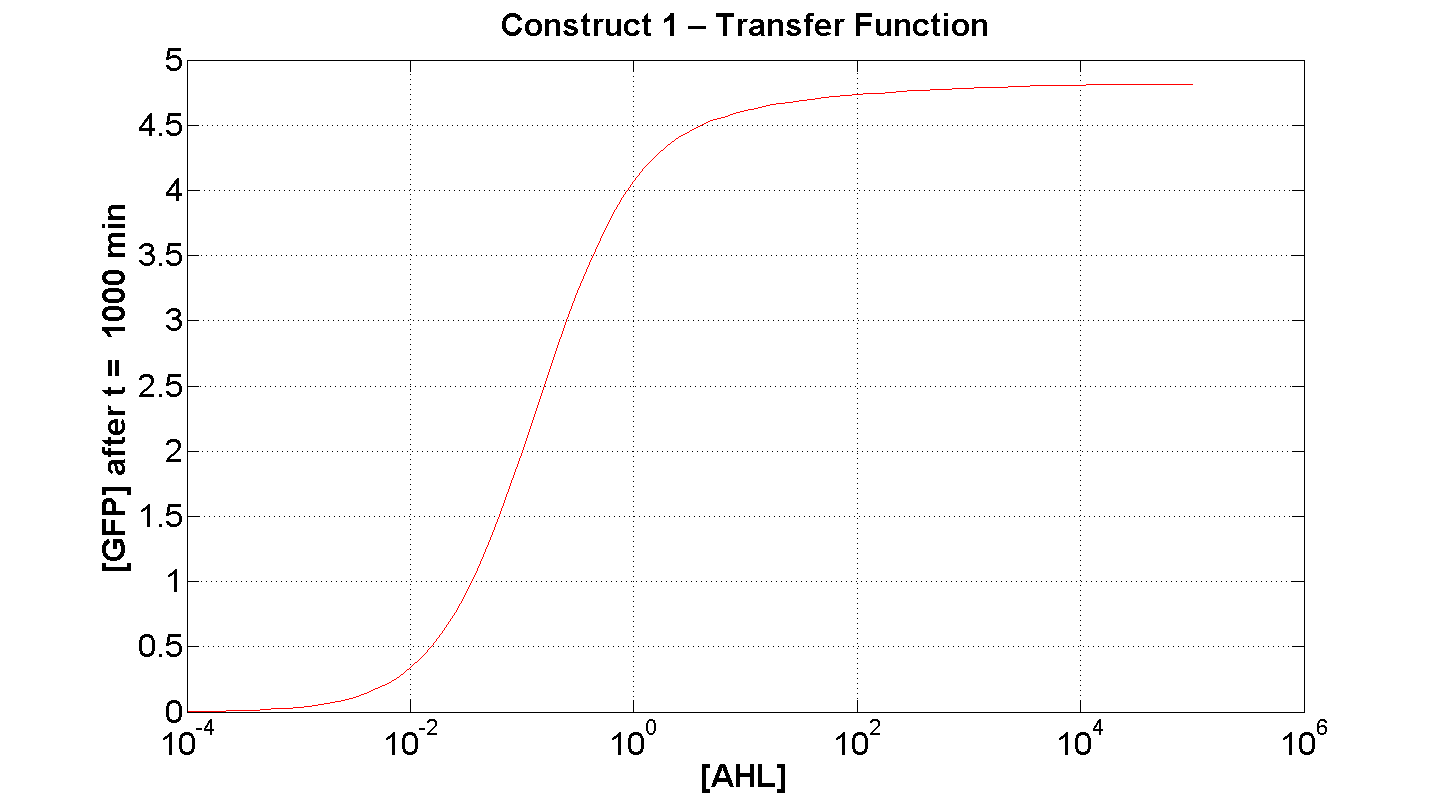
2. Construct 1: [GFP] vs [AHL] - transfer function curve
Discussion
Figure 7 serves to amplify the essential claims from the previous simulation. Saturative behaviour occurs at approximately [AHL] = 5-10 arbitrary units (a.u.). The effect of increasing initial [AHL] beyond this level is of negligible effect on [GFP] expression, for initially available resources. This first transfer function is taken at t=300min. We would expect the curve to be considerably reduced at a later instance. This is due to reduced energy resources. This ties in with what is observed in figure 8, at t = 1000 above: significantly reduced peak expression of GFP.
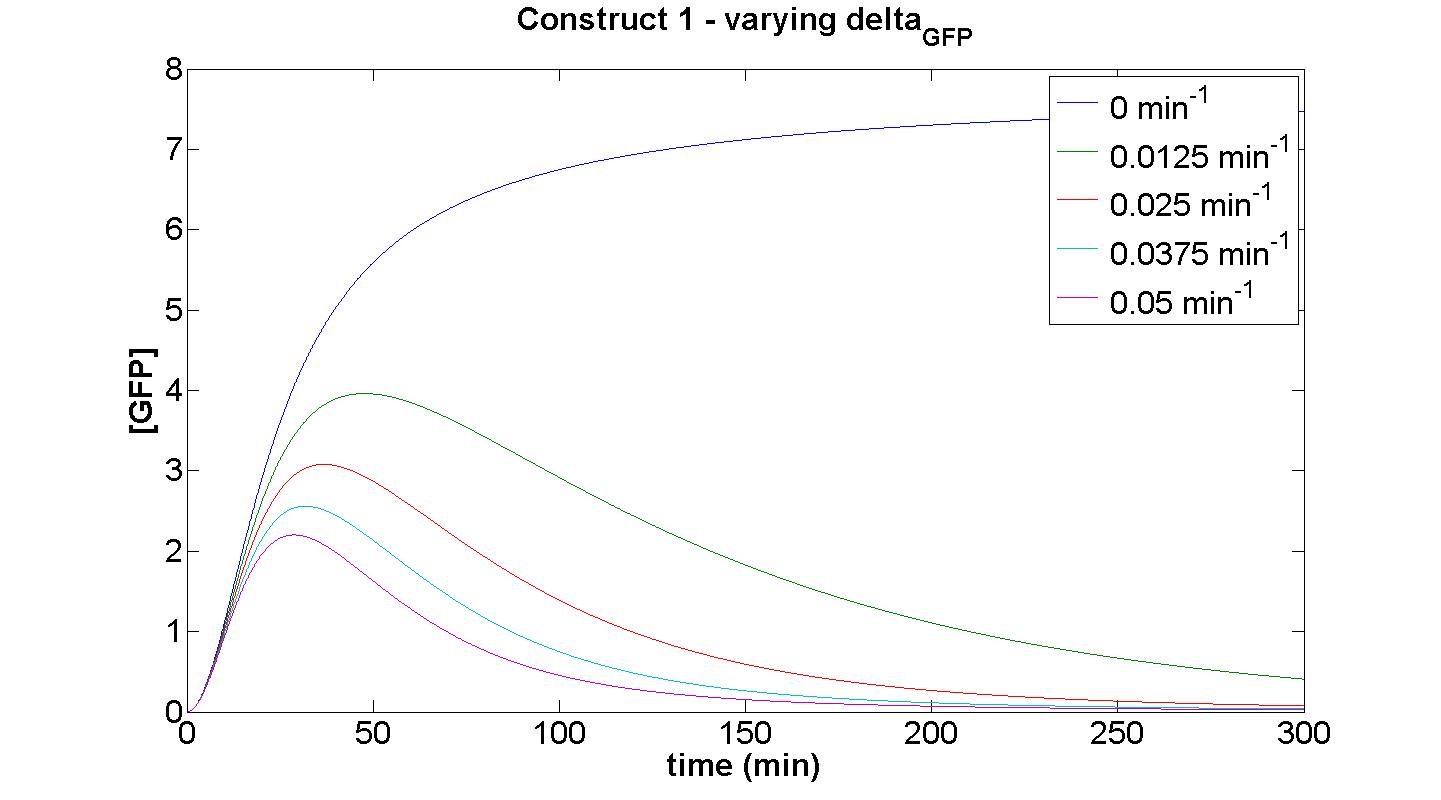
Simulation 3 – effect of varying GFP degradation term on dynamic behaviour of construct 1
Discussion
This simulation illustrates the effect of delta_GFP on the expression of GFP by construct 1. At small, delta_GFP, the steady-state approach clearly can be attributed to the considerable rate of energy depletion. When the degradation terms are more significant, their effect is considerably greater than the effect of limited resources. If our model were not energy-dependent, then the simulation for δGFP tending to zero should yield positively sloped parabolic curve.
Conclusions
From the initial simulations performed, it was suggested that construct 2 was more effective than construct 1 on the basis of sensitivity, response time and maximal output of reporter. Construct 1 was superior in terms of energy-efficiency. Its life-time is far greater than that of its counterpart.
Experimental work was performed on both constructs. The notable feature of these endeavours was that Construct 2 was eventually aborted due to difficulty in effectively purifying LuxR. Since our initial simulations are promising with regards to this device, it should be investigated more fully in the future. In any case, the simulations for construct 1, tie in with that presented by the experimental team, and are a fullfilment of our specifications.
Software
All deterministic simulations were performed using Matlab 7 (The MathWorks Inc., Natick, MA).
- m-files of all simulations are available on our Software page.