|
|
| (21 intermediate revisions not shown) |
| Line 82: |
Line 82: |
| | </td> | | </td> |
| | <td width=99> | | <td width=99> |
| - | <a href="lead"><image src="https://static.igem.org/mediawiki/2007/2/2a/Lead_reg.gif" alt="Lead Sensor" border=0></a> | + | <a href="lead"><image src="https://static.igem.org/mediawiki/2007/5/57/Lead_pressed.gif" alt="Lead Sensor" border=0></a> |
| | </td> | | </td> |
| | <td width=99> | | <td width=99> |
| - | <a href="tristable"><image src="https://static.igem.org/mediawiki/2007/7/79/Tri_pressed.gif" alt="Tristable Switch" border=0></a> | + | <a href="tristable"><image src="https://static.igem.org/mediawiki/2007/c/c5/Tri_reg.gif" alt="Tristable Switch" border=0></a> |
| | </td> | | </td> |
| | <td width=99> | | <td width=99> |
| Line 109: |
Line 109: |
| | | | |
| | | | |
| | + | =[[\Lead Detection | Lead Detection]]= |
| | + | According to the WHO, 40% of the planet does not have access to clean water. Lead is a serious contaminant around the world. It can cause neurological disorders, anemia, and death. In the United States alone, 3.5 million people have high levels of lead in their blood, so the problem is prevalent in both the developed and developing world. Children under the age of 12 are most at risk for lead poisoning. For more on lead poisoning, click [http://www.aquasanastore.com/water-you_c04.html here]. |
| | | | |
| - | =[[/Intro to Tristable | Tri-Stable Toggle Switch]]=
| + | [[Image:Inspiration.jpg]] |
| - | [[Image:Tristable_Toggle_Switch_2007.jpg|thumb|right]]A trinary memory unit. A genetic circuit. A proof of concept. Here is the man-made architecture of the switch and the natural context of our parts. | + | |
| | | | |
| | + | =[[\Design | Design]]= |
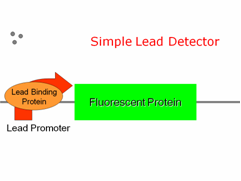
| | + | Having found a Lead Binding Protein and its corresponding Lead Promoter in the literature, we began to generate a number of ideas for our lead detector design. We began with a simple detector: The lead promoter could be placed in front of Green Fluorescent Protein, GFP. Once lead enters the cell, it activates the Lead Binding Protein, which activates our Lead Promoter, which in turn gives us some GFP output. But we realized that we could make this simple detector much more effective with an amplification circuit. |
| | | | |
| | + | [[Image:Lead Switch.jpg]] |
| | | | |
| | | | |
| | + | =[[\Experimental Steps | Experimental Steps]]= |
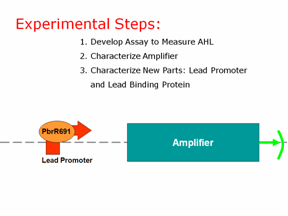
| | + | We have three experimental steps to create our final product: |
| | + | 1. Develop an assay to measure AHL |
| | + | 2. Characterize our amplifier |
| | + | 3. Characterize our new parts: the lead promoter and the lead binding protein |
| | + | [[Image:Experiment.jpg]] |
| | | | |
| | + | |
| | | | |
| | | | |
| | | | |
| | | | |
| | + | <html> |
| | + | </td> |
| | + | </tr> |
| | + | </table> |
| | + | </td> |
| | + | </tr> |
| | + | </table> |
| | + | </td> |
| | + | <td style="padding: 5px;" width=255 bgcolor="#e8e8e8" valign=top align=left> |
| | + | <img src="http://partsregistry.org/wiki/images/d/db/Buttonaddpart.gif"> |
| | + | </html> |
| | | | |
| | + | <strong>Parts added to the Registry:</strong> |
| | | | |
| - | =[[/Modeling | Tri-Stable Model]]=
| + | [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Brown Lead Detection Biobricks] |
| - | [[Image:beta values.png|thumb|right]]While Gardner et al created a mathematical model and a genetic switch in the Bistable paper, one was not used to design and improve upon the other. Here we extend our model to the Tristable system and discuss what certain parameters mean in terms of DNA and Proteins, Production, Repression, etc.
| + | |
| | | | |
| | + | <strong>Fully characterized 2 existing parts:</strong> |
| | | | |
| | + | [http://partsregistry.org/Part:BBa_T9002:Experience AHL to GFP Converter (BBa_T9002)] |
| | | | |
| - | | + | [http://partsregistry.org/Part:BBa_J37015:Experience Amplifier by Imperial College (BBa_J37105)] |
| - | | + | <html> |
| - | | + | </td> |
| - | | + | </tr> |
| - | | + | </table> |
| - | | + | </html> |
| - | =[[/Testing Constructs | Testing Constructs]]=
| + | |
| - | [[Image:betaTest.png|thumb|right]]The dirty side of the switch. Testing constructs that will enable us to determine our constants in absolute terms and apply a mathematical basis to changes we make on the Tri-Stable Switch architecture.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | =[[/Appendix | Appendix]]=
| + | |
| - | More information about where we are going and where we have been.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | <!--
| + | |
| - | =Tri-stable Toggle Switch=
| + | |
| - | The Tri-Stable switch three distinct and stable outputs in response to three distinct inputs. These three inputs are three separate chemicals which will each induce one state of the switch. [[Image:Tristable_Toggle_Switch_2007.jpg|thumb|left|The Tri-stable Toggle Switch Architecture]] In order to achieve this goal, we are constructing three constructs, each of which consists of a repressible, constitutively-on promoter attached to two repressors. Specifically, our three constructs are:
| + | |
| - | pBAD->LacI->TetR,
| + | |
| - | | + | |
| - | pLacI->AraC->TetR and
| + | |
| - | | + | |
| - | pTet->AraC->LacI,
| + | |
| - | | + | |
| - | where [http://en.wikipedia.org/wiki/L-arabinose_operon AraC] represses pAraC/BAD, [http://en.wikipedia.org/wiki/Lac_repressor LacI] represses pLac and [http://en.wikipedia.org/wiki/Tetracycline_controlled_transcriptional_activation TetR] represses pTet.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | Each of the three repressors are inactivated by one of three chemicals, the three inducer chemicals mentioned earlier. These three([http://en.wikipedia.org/wiki/Arabinose arabinose], [http://en.wikipedia.org/wiki/IPTG IPTG] (Isopropyl β-D-1-thiogalactopyranoside) and [http://en.wikipedia.org/wiki/Tetracycline Tetracycline], respectively), cause conformational changes in their respective repressor proteins which keeps them from binding to DNA in an inhibitory manner which leads to gene expression. For example, in the presence of arabinose, AraC cannot repress pAraC/BAD so LacI and TetR are produced which in turn repress pTet and pLac and the pAraC/BAD construct is turned on.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | ==AraC/BAD==
| + | |
| - | The gene AraC, one of several genes (AraA, AraB, AraD, etc) originally for the metabolism of arabinose.[http://www.mun.ca/biochem/courses/3107/Topics/Ara_operon.html]
| + | |
| - | [[Image:Two_Dimers_of_AraC.jpg|thumb|left|Dimer structure with arabinose on the left (yellow)]]
| + | |
| - | [[Image:AraC_Promoters.gif|left|thumb|The left image shows the araC dimer repressing transcription, while the right conformation enables transcription]]The protein forms a dimer with and without arabinose but the structural change activates or represses the pAraC/BAD.
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | ==LacI==
| + | |
| - | In nature, LacI represses pLac which promotes the LacYZA genes that metabolize lactose. Thus LacI represses pLac except in the presence of lactose (or lactose mimics, eg IPTG). [[Image:LacI_repressor.gif|thumb|left|Image[http://www.mun.ca/biochem/courses/3107/Topics/Lac_genetics.html]. LacI forms a tetramer and represses pLac. However, an inducer, such as IPTG, causes a conformation change that removes LacI from the operator site.]] Lactose causes a conformational change which inhibits LacI from binding to the operator site of pLac. Four LacI proteins form a tetramer to inhibit pLac and four inducer molecules are required to cause the full conformational change in the repressor.[http://www.mun.ca/biochem/courses/3107/Topics/Lac_genetics.html]
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | ==TetR==
| + | |
| - | TetR represses the constitutive promoter pTet. In the presence of tetracycline, an antibiotic, a conformational change in TetR inhibits the protein from binding to the operator region. In nature, pTet promotes TetR and TetA. The latter of which acts to pump tetracycline out of the cell, thus the pump is only activated in the presence of Tetracycline.[http://en.wikipedia.org/wiki/Tetracycline_controlled_transcriptional_activation]
| + | |
| - | The TetR, as it turns out is a very tight repressor and a range of 0 to 1 ug/ml has been shown to cause a 5 order of magnitude change in luciferase production.[http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=1319065&query_hl=1&itool=pubmed_docsum]
| + | |
| - | [[Image:Tc_bound_to_TetR.jpg|thumb|left|A tetracycline molecule binds to each of the two TetR monomers to form a dimer]]
| + | |
| - | | + | |
| - | =Modeling=
| + | |
| - | The first draft of the code started last year:
| + | |
| - | | + | |
| - | [http://parts2.mit.edu/wiki/index.php/Table_of_preliminary_model_constants Initial Table of Constants]
| + | |
| - | | + | |
| - | [http://parts2.mit.edu/wiki/index.php/Derivation_of_the_Model_Equations Derivation of Model Equations]
| + | |
| - | | + | |
| - | [[Media:tristable2006.txt]] This code proved to complicated to work with and a more simplified version was developed.
| + | |
| - | | + | |
| - | A simpler model based on the bistable paper was developed that takes the combined, relative transcription/translation rates into account [[Media:tristable2007.txt]].
| + | |
| - | ==Parameters in the Model==
| + | |
| - | ===Repressor Production Rate===
| + | |
| - | Repressor production rate is determined by two factors:
| + | |
| - | #Promoter Strength (Transcription)
| + | |
| - | #Ribosome Binding Strength (Translation)
| + | |
| - | | + | |
| - | In the model, the total repressor production rate = alpha
| + | |
| - | | + | |
| - | The promoter strength cannot be easily changed because this would require mutations in the promoter or a different promoter/repressor combo. However, RBSs have been well characterized and the alpha parameter can be modulated by inserting different strength RBSs as determined by the model and testing.
| + | |
| - | | + | |
| - | ===Repressor Strength===
| + | |
| - | The strength of the repressors is determined by:
| + | |
| - | #The repressor concentration, [Repressor].
| + | |
| - | #The cooperativity of repression, Beta.
| + | |
| - | | + | |
| - | In the Model, the repressor str
| + | |