Paris/DesignProcess
From 2007.igem.org
Contents |
Design Process
A first question that needs to be addressed in the design process of our system is that of the nature of the dependency relationships between its cellular components. Namely, what cross-dependency relationship between the somatic & germ line cells can best insure “ecological” equilibrium & “evolutionary” stability between the two cell types?
The soma needs the germ line
What dependency relationship?
A radical mean of ensuring somatic cell dependence on germ line is to make somatic cells sterile. That is, if somatic cells are unable to replicate, then they exclusively depend on G=>S differentiation for their existence. Furthermore, in this way it is impossible for S cells to overtake the population.
How to get sterility?
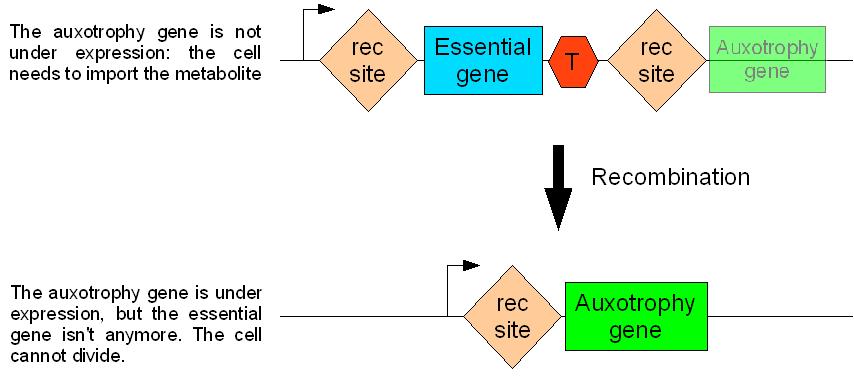
A great number of essential genes have been identified since the early days of E.coli genetics. An essential gene is one for which a mutant strain cannot grow at 37° on rich medium (LB). These genes include the essential division genes. Mutants of this genes cannot divide. Suppressing the expression of such a gene would thus make the cell sterile.
Differentiation mechanism
As discussed above, differentiation can be epigenetic (changes in genes expression pattern) or genetic (change in DNA sequence). In order to suppress the expression of an essential gene, we could either place it under the control of an inducible promoter that we would repress (epigenetic differentiation), or remove the gene itself through recombination (genetic differentiation). A genetic mechanism of differentiation is probably more robust than an epigenetic one in a way that the system artificially implemented in the cell should escape with more difficulties.
Therefore, we decided that the Germen=>Soma differentiation mechanism should be genetic (as opposed to epigenetic). Sterility in S cells will be achieved by deleting an essential gene from the genome during the differentiation process. This will be done through site-specific recombination.
Constraints on essential gene choice
Remains the question of the choice of the essential gene to be used. We have decided to base our choice on two criterions:
- Longevity of the S cells: S cells even is unable to divide should be able to live as long as possible. (an explanation for this is given here)
- Gene isolation: In our final construct, cassettes will be inserted before and after the essential gene. We want our gene to be isolated, so as not to disturb the expression of other nearby genes. It should not for instance be embedded in an operon. See here for more details about the construction method.
We have finally chosen ftsK as the gene to be used. It is independent of any operon. Also, ts strains for this gene give filamentous cells. The cells continue to grow without dividing until they die. This can go on for more than 10 generations, and the cells can reach impressive sizes.
The germ line needs the soma
What dependency relationship?
In our synthetic organism, the germ line is auxtroph for a given nutriment that is provided by the soma. In this way, a part of the germ line needs to differentiate, in order to feed the other part.
Why an auxotrophy dependence?
Previous works have shown that two types of cell, each auxotroph for a different metabolite, can rescue each other when grown in the same minimum media. This means that some auxotrophy should be rescued in a coculture with prototroph cells. Plus, it is very easy to do!
What auxotrophy?
In order to choose our auxotrophy, we need to take several constraints into account:
- No simple bypass or reversion: The auxotrophy phenotype of the germ line must be stable. There must be no simple metabolic bypass or reversion possibilities.
- Overproduction and excretion: Prototroph cells must be able to overproduce and excrete the metabolite, or at least to release it when dying.
- Absorption: auxotroph cells should be able to live in very low concentration of this metabolite. Indeed we should expect only little metabolite to be excreted.
- Survival: auxotroph cells should be able to live as long as possible when deprived of the metabolism.
- No growth on LB: We would like our synthetic organism to be able to grow on rich medium. This would facilitate our lab work, and more generally allow our synthetic organism to grow in a variety of conditions.
- Feedback: For purposes described below, we would like to have a sensor device sensitive to our metabolite concentration.
We finally choose diaminopimelate (DAP) as our metabolite. The germ line will be dapA-. For details about our choice see here . In order to confirm that our choice fits well the above mentioned criteria, we conducted several experiments:
- dapA- strains viability
- dapA- strains growth on limited DAP concentration
- DAP excretion measurments
- Coculture experiments
How will auxotroph germ line cells differentiate into prototroph somatic cells?
We found a simple way to solve this problem. This is best described by the following schema.
System scale specifications
What differentiation rate?
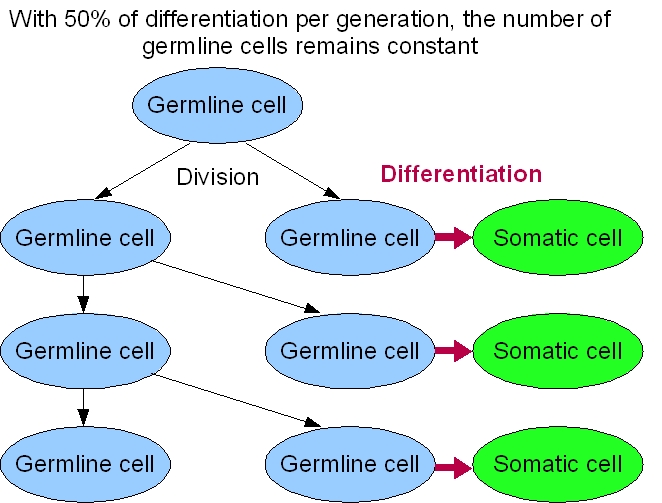
Our system sucess rely a lot on the differentiation rate of the germ line into soma. A simple fact, is that we cannot have more than 50% of differentiation per generation. Otherwise, the germline will only decrease. But this not the only constraint to take into account.
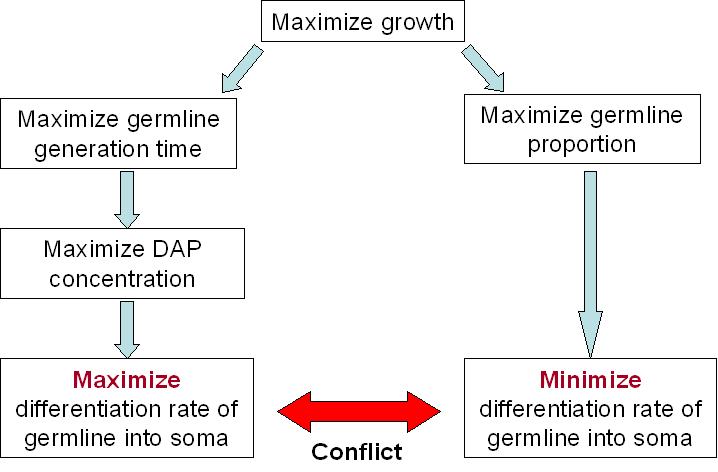
For our germ line to live best, we need to maximize the DAP production. This means that we need to maximize the excretion of DAP by the soma and to maximize the proportion of the soma itself. The number of somatic cells is given by the differentiation rate of germ line cells and the life time of somatic cells. Thus we need to maximize the life time of somatic cells and the differentiation rate of the germ line.
But on the other end, we want our synthetic organism to grow as fast as possible. And it will grow faster if the proportion of germ line cells is bigger, since somatic cells do not divide. This means that we have to minimize the differentiation rate.
We clearly see the conflict here, which will ultimately lead to a tradeoff between the germ line proportion and its generation time. As explained above, the differentiation rate cannot be over 50% per generation if we want our synthetic organism to grow, but what this tradeoff tells us, is that there is an optimum differentiation rate somewhere between 0 and 50% recombinant per generation. This is best illustrated by the modeling of our system.
What recombination system?
According to the previous point, we need a recombination system whose rate we will be able to control. Ideally we want to be able to achieve recombination at any chosen frequency, but if we can’t do this, we need at least to be able to have a recombination rate below 50% per generation but high enough for the germ line to be sufficiently fed. Another constraint on the recombination system, is that we want the differentiation to be unidirectional. We chose the Cre/Lox recombination system, for the following reasons:
- it is readily available, largely used and well described
- lox66 and lox71 recombination sites have been described to produce unidirectional recombination ( Cre recombinase-mediated inversion using lox66 and lox71: method to introduce conditional point mutations into the CREB-binding protein, Zuwen Zhang and Beat Lutz, Nucleic Acids Research)
We want to control the recombination frequency by adjusting the expression strength of the Cre recombinase. To do this, we cloned the Cre recombinase under the control of the pBad promoter (inducible through arabinose). We also decided to clone our Cre production device (araC-pBad-rbs-Cre) on a low copy number plasmid. To see the measurements of recombination rates go here.
Optimization through feedback
It is clear that the average recombination rate must be between 0 and 50% per generation. Nevertheless, we can theoretically maximize the growth rate by adjusting the recombination rate to the DAP starvation as shown by our models. In this way, the germ line would increase its differentiation rate when it lacks DAP, and reduce it when there is enough DAP. In order to achieve this, we need to be able to adjust the Cre production to the DAP concentration. This would be easily dine if there where a promoter sensitive to DAP concentration, which seems to be the case of the dapA promoter (dapAp). We therefore cloned and characterized this promoter.
Overview of the project
(Flash animation J-3)