Paris/Project Description
From 2007.igem.org
David.bikard (Talk | contribs) (→Tradeoff) |
David.bikard (Talk | contribs) (→System scale specifications) |
||
| Line 90: | Line 90: | ||
===System scale specifications=== | ===System scale specifications=== | ||
| + | |||
| + | ===What differentiation rate?=== | ||
| + | |||
| + | Our system sucess rely a lot on the differentiation rate of the germline into soma. A simple fact, is that we cannot have more than 50% of differentiation per generation. Otherwise, the germline will only decrease. But this not the only constraint to take into account. | ||
For our germline to live best, we need to maximize the DAP production. This means that we need to maximize the excretion of DAP by the soma and to maximize the proportion of the soma itself. The number of somatic cells is given by the differentiation rate of germline cells and the life time of somatic cells. Thus we need to maximize the life time of somatic cells and the differentiation rate of the germline. | For our germline to live best, we need to maximize the DAP production. This means that we need to maximize the excretion of DAP by the soma and to maximize the proportion of the soma itself. The number of somatic cells is given by the differentiation rate of germline cells and the life time of somatic cells. Thus we need to maximize the life time of somatic cells and the differentiation rate of the germline. | ||
Revision as of 15:40, 14 October 2007
This page is under construction
The idea is to make a multicellular organism out of bacteria. This simple organism will have two distinct types of cells. The first one would be the germline, the other one the soma. The germline will be auxotroph for a given nutriment, which will be provided by the soma. The soma will differentiate from the germline and will be unable to divide. In this way, there is a need for a part of the germline to differentiate into soma in order to feed the rest of the germline. Hence, the soma doesn't exist without a germline to generate it.
Contents
|
Introduction
Why a Synthetic Multicellular Bacterium ?
Dealing with complexity
A major challenge for synthetic biology will be to tackle complexity. If you want to modify an organism so that it can perform complex tasks, may wish to implement a complex circuit within a single cell. But, in order to do this, you would require very well characterized biobricks if you want to make sure that your system will behave as predicted. And even if this is the case you may stumble upon unexpected interactions. A smarter approach might be to implement small simple parts of your circuit within different types of cells that would work together. The aim of our project is to provide a tool for such an approach, making a jump in complexity scale, from single cells to multicellular synthetic biology, and for the first time engineering a multicellular bacterium.
Avoiding interactions
For the moment only very few parts in the registry are well characterized. This leads to the fact that almost all the devices are made out of the same biobricks (plac, pBad, ptet, plambda, luxI, luxR…), and are thus incompatible (you cannot implement them in the same cell). Implementing them in different cell types could solve this problem…
A new tool for synthetic biology and metabolic engineering
In most multicellular organism there is a separation between the cell line devoted to reproduction, the germline, and the one devoted to support the germline and increase its likeliness of reproduction, the soma. The particularity of somatic cells, is that in a sense they sacrifice themselves for the benefit of the organism. In the same way, implementing a differentiation between a soma and a germline in a bacteria, could allow using the soma to produce compounds noxious for itself. In this perspective, our multicellular organism could become a great tool for metabolic engineering.
What is a multicellular organism and what do we need create one ?
What will our synthetic multicellular organism look like?
Design Process
A first question that needs to be addressed in the design process of our system is that of the nature of the dependency relationships between its cellular components. Namely, what cross-dependency relationship between the somatic & germline cells can best insure “ecological” equilibrium & “evolutionary” stability between the two cell types?
The soma needs the germline
What dependency relashioship?
A radical mean of ensuring somatic cell dependence on germ line is to make somatic cells sterile. That is, if somatic cells are unable to replicate, then they exclusively depend on GS differentiation for their existence. In absence of S cell proliferation, G cells can not be pushed to extinction by S growth.
How to get sterility?
A great number of essential genes have been identified since the early days of E.coli genetics. An essential gene is one for which a mutant strain can not grow at 37° on rich medium (LB). These genes are often studied using “ts” (thermo-sensitive) alleles. Meaning alleles that allow growth only at low temperature (usually 30°).
Differentiation mechanism
The genetic network of a cell is finely regulated and not so much understood. A genetic mechanism of differentiation also seemed to be more robust than epigenetic one in a way that the system artificially implemented in the cell should escape with more difficulties. As mentioned above, we have decided that the Germen=>Soma differentiation mechanism would be genetic (as opposed to epigenetic). Sterility in S cells will be achieved by deleting an essential gene from the genome during the differentiation process. As discussed below, site specific recombination is an appropriate mechanism by which this can be performed.
Constraints on essential gene choice
Remains the question of the choice of the essential gene to be used. We have decided to base our choice on two criterions:
- Longevity of the S cells: S cells even is unable to divide should be able to live as long as possible. (an explanation for this is given in section …..)
- Gene isolation: In our final construct, cassettes will be inserted before and after the essential gene. We want our gene to be isolated, so as not to disturb the expression of other nearby genes. It should not for instance be embedded in an operon. See here for more details about the construction method.
We have finally chosen ftsK as the gene to be used. It is independent of any operon. Also, ts strains for this gene give filamentous cells. The cells continue to grow without dividing until it dies. This can go on for more than 10 generations, and the cells can reach impressive sizes.
The Germline needs the soma
What dependency relationship?
In our synthetic organism, the germline is auxtroph for a given nutriment that is provided by the soma. In this way, a part of the germline needs to differentiate, in order to feed the other part.
Why an auxotrophy dependence?
Previous works have shown that two types of cell, each auxotroph for a different metabolite, can rescue each other when grown in the same minimum media. This means that some auxotrophy should be rescued in a coculture with prototroph cells. Plus, it is very easy to do!
What auxotrophy?
In order to choose our auxotrophy, we need to take several constraints into account:
- No simple bypass or reversion: The auxotrophy phenotype of the germline must be stable. There must be no simple metabolic bypass or reversion possibilities.
- Overproduction and excretion: Prototroph cells must be able to overproduce and excrete the metabolite, or at least to release it when dying.
- Absorption: auxotroph cells should be able to live in very low concentration of this metabolite. Indeed we should expect only little metabolite to be excreted.
- Survival: auxotroph cells should be able to live as long as possible when deprived of the metabolism.
- No growth on LB: We would like our synthetic organism to be able to grow on rich medium. This would facilitate our lab work, and more generally allow our synthetic organism to grow in a variety of conditions.
- Feedback: For purposes described below, we would like to have a sensor device sensitive to our metabolite concentration.
We finally choose diaminopimelate (DAP) as our metabolite. The germline will be dapA-. For details about our choice see here. In order to confirm that our choice fits well the above mentioned criteria, we conducted several experiments.
How will auxotroph germline cells differentiate into prototroph somatic cells?
We found a simple way to solve this problem. This is best described by the following schema.
System scale specifications
What differentiation rate?
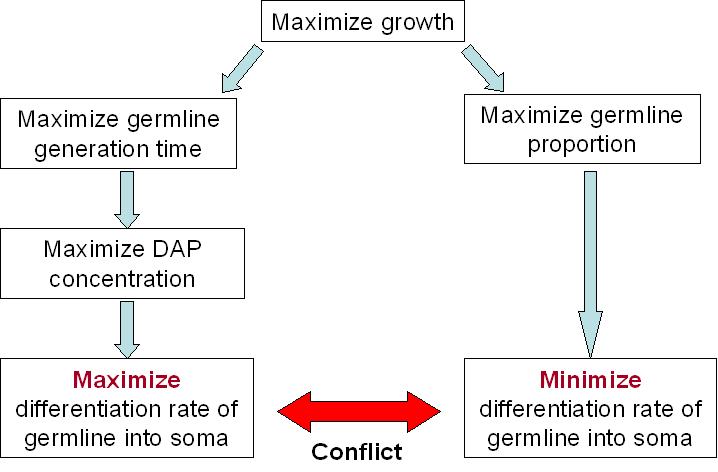
Our system sucess rely a lot on the differentiation rate of the germline into soma. A simple fact, is that we cannot have more than 50% of differentiation per generation. Otherwise, the germline will only decrease. But this not the only constraint to take into account.
For our germline to live best, we need to maximize the DAP production. This means that we need to maximize the excretion of DAP by the soma and to maximize the proportion of the soma itself. The number of somatic cells is given by the differentiation rate of germline cells and the life time of somatic cells. Thus we need to maximize the life time of somatic cells and the differentiation rate of the germline. But on the other end, we want our synthetic organism to grow as fast as possible. And it will grow faster if the proportion of germline cells is bigger, since somatic cells do not divide. This means that we have to minimize the differentiation rate.
We clearly see the conflict here, which will ultimately lead to a tradeoff between the germline proportion and the germline generation time. As explained above, the differentiation rate cannot be over 50% per generation if we want our synthetic organism to grow, but what this tradeoff tells us, is that there is an optimum differentiation rate somewhere between 0 and 50% recombinant per generation. This is best illustrated by the modeling of our system.
What recombination system?
According to the previous point, we need a recombination system whose rate we will be able to control. Ideally we want to be able to achieve recombination at any chosen frequency, but if we can’t do this, we need at least to be able to have a recombination rate below 50% per generation but high enough for the germline to be sufficiently fed. Another constraint on the recombination system, is that we want the differentiation to be unidirectional. We chose the Cre/Lox recombination system, for the following reasons:
- it is readily available, largely used and well described
- lox66 and lox71 recombination sites have be described to produce unidirectional recombination (ref)
We want to control the recombination frequency by adjusting the expression strength of the Cre recombinase. To do this, we cloned the Cre recombinase under the control of the pBad promoter (inducible through arabinose). We also decided to clone our Cre production device (araC-pBad-rbs-Cre) on a low copy number plasmid. To see the measurements of recombination rates go here.
Optimization through feedback
It is clear that the average recombination rate must be between 0 and 50% per generation. Nevertheless, we can theoretically maximize the growth rate by adjusting the recombination rate to the DAP starvation as shown by our models. In this way, the germline would increase its differentiation rate when it lacks DAP, and reduce it when there is enough DAP. In order to achieve this, we need to be able to adjust the Cre production to the DAP concentration. This would be easily dine if there where a promoter sensitive to DAP concentration, which seems to be the case of the dapA promoter (dapAp). We therefore cloned and characterized this promoter.
Overview of the project
Applications
Metabolic engineering
E. Colight: optimization of triglycerides synthesis in the soma of the synthetic organism: towards a new slim diet
A bacterial multicellular organism could allow optimizing the production of compounds deleterious to the cell. If you try to optimize the production of such a compound in a classic bacteria (E.coli for instance), you will reach a trade off between the production of your compound and the growth of the cell because the reproduction capacity is linked to the expression of the toxic transgene. The synthetic organism could in part bypass this problem, decoupling both basis functions. It could indeed be modified so that the soma only will produce the noxious molecule and the germline will multiply. If there is a way to screen for the production of this molecule, we can then select the germline whose soma would have the best yield. As long as the production doesn't impair to much the capacity of the soma to feed the germline, the optimization can go on. Thus, there is also a trade off in this case, but it might very well be more favorable to the optimization than the trade off between production and growth. At least, this is worth testing. Of course, all this is only possible if the germline is not affected by the production of the soma. Compounds that could be optimized in this way are thus constrained to molecules that can be noxious in the cell, but that do not affect it to much if in the medium. Finally, the last part of our project would be to bring together the synthetic organism, the optimization of a compound noxious to the bacteria, and the security device. To do so, we'd like to optimize the production of triglycerides in the soma cells of our synthetic organism. We would then be able to differentiate all the germline of the synthetic organism into soma in a secured way. Those super triglycerides-producing-not-able-to-divide cells could then be ingested. The fatty acids they would stock would be as many fatty acids you will not absorb! Eat fat, don't get fat ! Triglycerides are made of a molecule of glycerol esterified by three fatty acids. When ingested they are hydrolysed by lipase in the stomach and the duodenum, into glycerol and free fatty acids. Enterocytes are only able to absorb free fatty acids and glycerol. So they combined them in the cytoplasm into triglycerides that will be free in the lymphatic system then in the blood within fatty vesicles called chylomicrons. When we are becoming fat, it is because we have more input than output energy, lipid energy. Knowing that gut is full with bacteria forming the gut microflora (we have 1013 of our cells in our body and 1014 bacteria with the majority in the gut!), we could imaging having a somatic cell bacteria able to absorb fatty acids and form triglycerides intracellular inclusion. These triglycerides are not able to be absorbed by enterocytes! Input lipid is decreased! Eat fat, don't get fat! E.coli is the most used bacterium in synthetic biology and... belongs to the gut microflora! We could also imagine genetically engineering E.coli to stock triglycerides into inclusions! Knowing that 40% of E.coli are renewed every day, these triglyceride-fulled bacteria will leave the gut with feces! With the security device described above we should have a safe GMO with two hours life expectancy!
Security device
secure isolation of the soma => release in the environment
We also came up with the idea of modifying the synthetic organism so that we would be able to induce the differentiation of all the germline into soma in a secured way. Why would we want to do that ? Well, in a security perspective, we may not want to release a transgenic strain in the environment. But it would be acceptable to do so if we are sure that the cells we release are not able to proliferate. In this view, we could release in the environment, what would correspond to our "soma". Those cells aren't able to divide and their effect is limited in time since they will die after approximatively 2h. Indeed, one of the problems with GMO is the selective advantage that the gene gives. If it is greater than for the wild type, the GMO will spread and replace the wild type in the ecosystem. For example, if we think about the gut flora, a genetic engineered E.coli could spread and replace the wild type E.coli causing diseases (diarrhoea...). That could be possible because the reproductive faculty is linked to the gene given: the gene gives a better reproductive capacity of the GMO. One way to avoid this problem is decoupling the reproduction faculty and the expression of the gene: the organism is either able to multiply or able to express the gene. So expressing the transgene can not be responsible of a selective advantage against the wild type.