Rice
From 2007.igem.org
| Line 6: | Line 6: | ||
** [[Rice/Team2007#Meeting Times|Meeting Times]] | ** [[Rice/Team2007#Meeting Times|Meeting Times]] | ||
** [[Rice/Team2007#Information For Prospective Members|Information For Prospective Members]] | ** [[Rice/Team2007#Information For Prospective Members|Information For Prospective Members]] | ||
| - | + | <BR><BR> | |
| - | + | <P> | |
==[[Rice/Project A: Phage Project|Project A: Phage Project Overview]]== | ==[[Rice/Project A: Phage Project|Project A: Phage Project Overview]]== | ||
<div style="float:right;width:315px;"> | <div style="float:right;width:315px;"> | ||
| Line 18: | Line 18: | ||
Following up on the elucidation of this phenomenon, our iGEM team asks the following question: '''Can we artificially engineer bacteriophage which would produce a selection against antibiotic resistance within bacterial populations?''' | Following up on the elucidation of this phenomenon, our iGEM team asks the following question: '''Can we artificially engineer bacteriophage which would produce a selection against antibiotic resistance within bacterial populations?''' | ||
</div> | </div> | ||
| + | </P> <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | ||
| + | |||
| + | <P> | ||
==[[Rice/Project B: Quorumtaxis Project|Project B: Quorumtaxis Project Overview]]== | ==[[Rice/Project B: Quorumtaxis Project|Project B: Quorumtaxis Project Overview]]== | ||
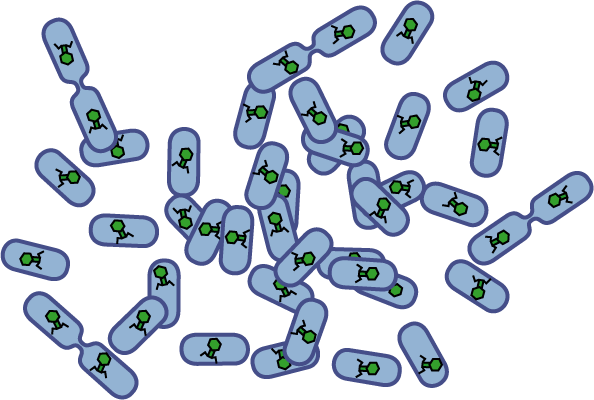
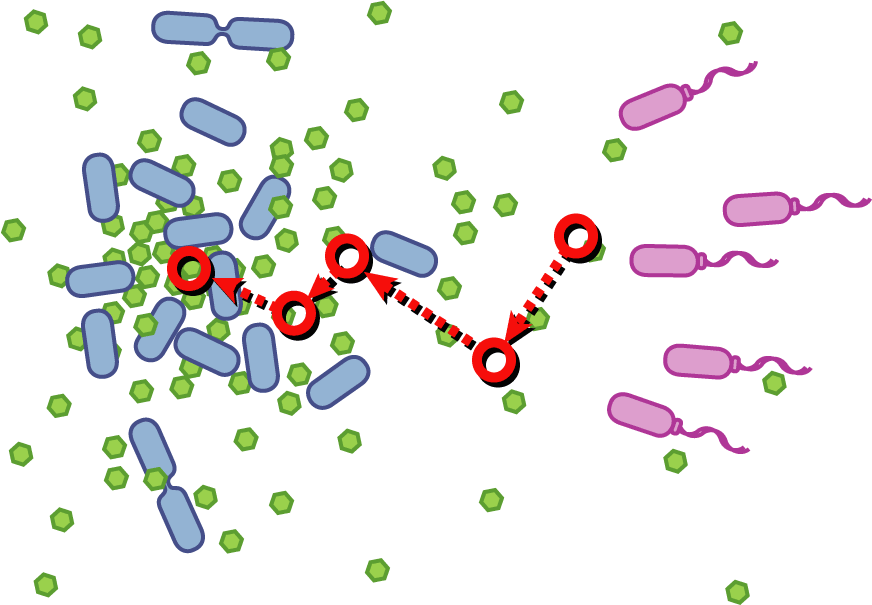
[[Image:Quorumtaxis.gif|thumb|left|450px|Quorumtaxis: The re-engineered ''E.coli'' (shown in purple) are able to detect gradients of quorum pheromones in the environment (green hexagons) produced by the target species (blue cells) and actively travel up this gradient.]] | [[Image:Quorumtaxis.gif|thumb|left|450px|Quorumtaxis: The re-engineered ''E.coli'' (shown in purple) are able to detect gradients of quorum pheromones in the environment (green hexagons) produced by the target species (blue cells) and actively travel up this gradient.]] | ||
| Line 24: | Line 27: | ||
Our iGEM team asks the following question: '''Can we re-engineer ''E.coli'' to recognize and pursue other bacterial species?''' | Our iGEM team asks the following question: '''Can we re-engineer ''E.coli'' to recognize and pursue other bacterial species?''' | ||
| + | |||
| + | </P> | ||
Revision as of 21:00, 23 October 2007
Welcome to the home of Rice University iGEM 2007 Team.
Project A: Phage Project Overview
Recently it has been demonstrated how differential selection between antibiotic resistance and sensitive bacterial populations is affected under a regime of multiple drug combinations. Normally, if two populations of the same bacterial strain compete, the one which mutated to acquire anti-biotic resistance 'wins' the fight for survival over the ones that remained wild-type and antibiotic sensitive. However, in certain drug combination regimens that involve suppressive interactions, high drug concentrations results in competitive selection against resistance, without perturbing the effectiveness of the other drug in the combination. These results are interpreted as coming from a trade-off regime in the evolution of resistance fitness landscape, where absolute potency of a drug combination is balanced by the relative competitive selection imposed on emerging resistant populations.
Following up on the elucidation of this phenomenon, our iGEM team asks the following question: Can we artificially engineer bacteriophage which would produce a selection against antibiotic resistance within bacterial populations?
Project B: Quorumtaxis Project Overview
Bacteria have evolved diverse genetic systems to sense their environment as well as respond to their surroundings in an adaptive manner. Of recent intense interest is the discovery that bacteria use pheromones that allow them to sense their own population density, a system known as quorum sensing. Another widely studied system is that of chemotaxis, in which a bacterium is able to sense and adaptively swim towards or away from a chemical agent in the environment. The Rice University iGEM team is attempting to merge these two existing natural systems (quorum sensing and chemotaxis) to produce a novel bacterial phenotype (quorumtaxis) in which the engineered cell will be able to detect and swim towards the quorum pheromone of another ‘target’ cell. This project will demonstrate the ability to produce unique complex behavior in bacteria through the modular integration of existing circuits. In addition, precise control over bacterial movement will greatly increase the complexity of systems synthetic biologists could create. The keystone of the project is the design of a novel chimeric receptor which can sense the target pheromone and then signal flagellar rotation within the cell. This project necessitates a highly interdisciplinary approach: the conceptual design and part construction requires backgrounds in biochemistry and cell biology, protein engineering elements requires experience in biomolecular engineering methods, and computational mathematics will be used to model the quorumtaxis phenotype.
Our iGEM team asks the following question: Can we re-engineer E.coli to recognize and pursue other bacterial species?