Rice
From 2007.igem.org
| (24 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | <BR> | |
| - | + | <div style="float:left;width:450px;"> | |
| - | + | [[Image:Ricetitle.jpg|none]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <div style="float: | + | |
| - | [[Image: | + | |
| - | + | ||
</div> | </div> | ||
| - | < | + | <P> |
| - | + | ||
| - | + | <BR><BR> | |
| - | </ | + | <font color="navy"> |
| + | '''Welcome to Rice University's 2007 iGEM wiki page. We have a number of incredible projects this year! Our new project is focused on the design a circuit to modulate phage-bacteria ecological dynamics towards the goal of selecting against antibiotic resistance. The continuation of last year's project involves the construction of a novel bacterial phenotype, quorumtaxis, in which bacteria are programmed to detect and pursue other bacterial species.''' | ||
| + | </font> | ||
| + | <BR><BR><BR> | ||
| + | =<BR><BR><BR><BR><BR>'''Project A: Phage that select against bacterial resistance'''= | ||
| + | [[Image:Phage_Figure1.gif|thumb|300px|right|'''A.''' Engineered phage infect cells, lying dormant as lysogens.]] | ||
| - | + | The widespread use of antibiotics has amplified the fraction of virulent microbes in the biosphere that have evolved genetic-encoded tricks to sidestep the toxic effects of these drugs. In fact, a recent study published in the Journal of the American Medical Association suggested that the number of deaths associated with Methicillin-Resistant ''Staphylococcus aureus'' (MRSA), exceeds those attributed to AIDS, Parkinson’s disease, emphysema or homicide each year (see [[JAMA 298:1763-1771]]). This ever-increasing problem stems from the fact that antibiotic use selects for bacteria with resistance and increases the fraction of total bacteria in the biosphere that are resistant. Bacterial populations have little or no selection pressure against them to change this enrichment of resistant cells in the absence of antibiotics. The goal of our genetic circuit is to program the fitness of antibiotic resistant cells to be lower than that of antibiotic sensitive cells in the presence of non-toxic levels of antibiotic. In this way, we seek to reduce the fraction of antibiotic resistant cells in the biosphere and increase the utility of existing antibiotics. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | [[Image:Phage_Figure2.gif|thumb|300px|right|'''B.''' When the presence of antibiotic X results in the increased expression of resistance genes, the phage enters the lytic phase and kills all X-resistant cells.]] | ||
| + | ===[[Rice/Project A: Phage Project|'''Details...''']]=== | ||
| + | </P> <BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR><BR> | ||
| - | + | <P> | |
| - | + | ='''Project B: Quorumtaxis'''= | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | = | + | |
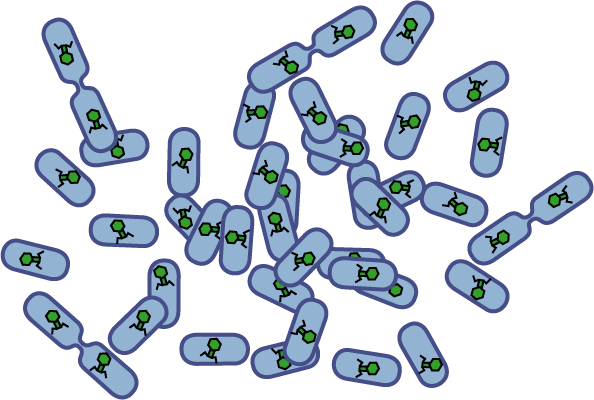
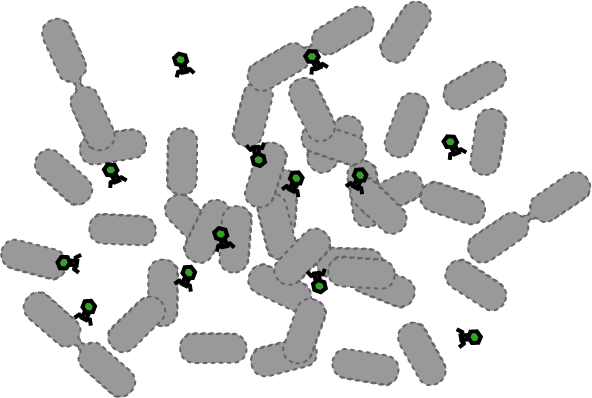
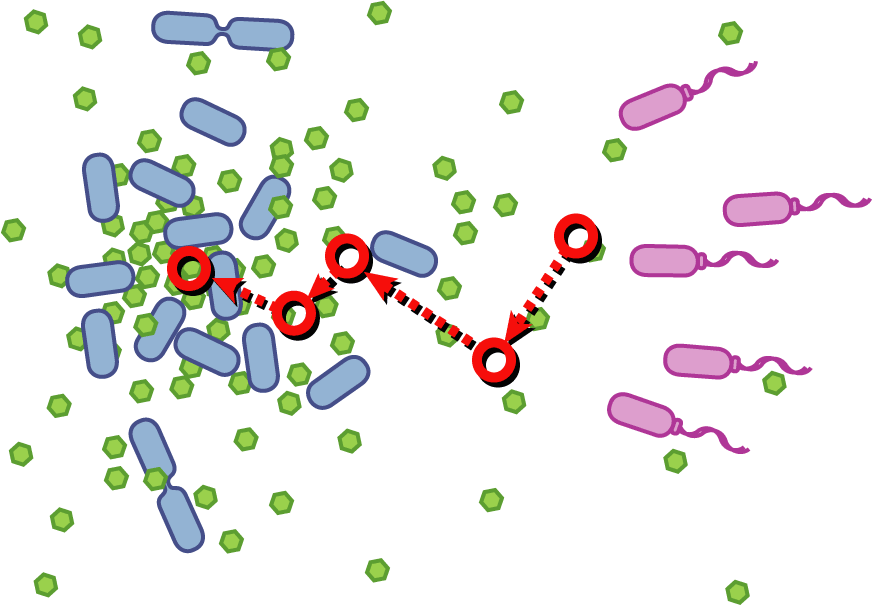
[[Image:Quorumtaxis.gif|thumb|left|450px|Quorumtaxis: The re-engineered ''E.coli'' (shown in purple) are able to detect gradients of quorum pheromones in the environment (green hexagons) produced by the target species (blue cells) and actively travel up this gradient.]] | [[Image:Quorumtaxis.gif|thumb|left|450px|Quorumtaxis: The re-engineered ''E.coli'' (shown in purple) are able to detect gradients of quorum pheromones in the environment (green hexagons) produced by the target species (blue cells) and actively travel up this gradient.]] | ||
Bacteria have evolved diverse genetic systems to sense their environment as well as respond to their surroundings in an adaptive manner. Of recent intense interest is the discovery that bacteria use pheromones that allow them to sense their own population density, a system known as quorum sensing. Another widely studied system is that of chemotaxis, in which a bacterium is able to sense and adaptively swim towards or away from a chemical agent in the environment. The Rice University iGEM team is attempting to merge these two existing natural systems (quorum sensing and chemotaxis) to produce a novel bacterial phenotype (quorumtaxis) in which the engineered cell will be able to detect and swim towards the quorum pheromone of another ‘target’ cell. This project will demonstrate the ability to produce unique complex behavior in bacteria through the modular integration of existing circuits. In addition, precise control over bacterial movement will greatly increase the complexity of systems synthetic biologists could create. The keystone of the project is the design of a novel chimeric receptor which can sense the target pheromone and then signal flagellar rotation within the cell. This project necessitates a highly interdisciplinary approach: the conceptual design and part construction requires backgrounds in biochemistry and cell biology, protein engineering elements requires experience in biomolecular engineering methods, and computational mathematics will be used to model the quorumtaxis phenotype. | Bacteria have evolved diverse genetic systems to sense their environment as well as respond to their surroundings in an adaptive manner. Of recent intense interest is the discovery that bacteria use pheromones that allow them to sense their own population density, a system known as quorum sensing. Another widely studied system is that of chemotaxis, in which a bacterium is able to sense and adaptively swim towards or away from a chemical agent in the environment. The Rice University iGEM team is attempting to merge these two existing natural systems (quorum sensing and chemotaxis) to produce a novel bacterial phenotype (quorumtaxis) in which the engineered cell will be able to detect and swim towards the quorum pheromone of another ‘target’ cell. This project will demonstrate the ability to produce unique complex behavior in bacteria through the modular integration of existing circuits. In addition, precise control over bacterial movement will greatly increase the complexity of systems synthetic biologists could create. The keystone of the project is the design of a novel chimeric receptor which can sense the target pheromone and then signal flagellar rotation within the cell. This project necessitates a highly interdisciplinary approach: the conceptual design and part construction requires backgrounds in biochemistry and cell biology, protein engineering elements requires experience in biomolecular engineering methods, and computational mathematics will be used to model the quorumtaxis phenotype. | ||
| Line 45: | Line 29: | ||
Our iGEM team asks the following question: '''Can we re-engineer ''E.coli'' to recognize and pursue other bacterial species?''' | Our iGEM team asks the following question: '''Can we re-engineer ''E.coli'' to recognize and pursue other bacterial species?''' | ||
| + | ===[[Rice/Project B: Quorumtaxis|'''Details...''']]=== | ||
| + | </P> | ||
| - | + | <BR> | |
| - | + | ---- | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | Thanks for visiting our wiki, and be sure to tell us what you think when we see you at the iGEM Jamboree! | ||
| + | <BR><BR> | ||
| + | <font color="navy"> | ||
| + | ='''2007 Team Roster'''= | ||
| + | [[Image:RiceSyntheticTitle.jpg|center]] | ||
| + | <BR><BR> | ||
| + | <div style= "position: absolute; left:500px"> | ||
| + | [[Image:Rice07Team.jpg|thumb|none|300px|Top: Thomas, Taylor, David. | ||
| + | Bottom: Joff, Peter, Beth, and Alec.]] | ||
| + | </div> | ||
| + | <div style= "position: center;"> | ||
| + | * '''Undergraduate Students''' | ||
| + | ** Tina Chen | ||
| + | ** Thomas Segall-Shapiro | ||
| + | ** Taylor Stevenson | ||
| + | ** David Kim | ||
| + | ** Alec Walker | ||
| + | **Arielle Layman | ||
| + | <BR> | ||
| + | * '''Graduate Advisors''' | ||
| + | ** Bibhash Mukhopadhyay | ||
| + | ** Peter Nguyen | ||
| + | ** Christie Peebles | ||
| + | ** Jay Raol | ||
| + | <BR> | ||
| + | * '''Faculty Advisors''' | ||
| + | ** Beth Beason, PhD. | ||
| + | ** George Bennett, PhD. | ||
| + | ** Steve Cox, PhD. | ||
| + | ** Ken Cox, PhD. | ||
| + | ** Ka-yiu San, PhD. | ||
| + | ** Joff Silberg, PhD. | ||
| + | </div> | ||
| + | </font> | ||
---- | ---- | ||
| - | |||
| - | |||
Latest revision as of 03:10, 27 October 2007
Welcome to Rice University's 2007 iGEM wiki page. We have a number of incredible projects this year! Our new project is focused on the design a circuit to modulate phage-bacteria ecological dynamics towards the goal of selecting against antibiotic resistance. The continuation of last year's project involves the construction of a novel bacterial phenotype, quorumtaxis, in which bacteria are programmed to detect and pursue other bacterial species.
Contents |
Project A: Phage that select against bacterial resistance
The widespread use of antibiotics has amplified the fraction of virulent microbes in the biosphere that have evolved genetic-encoded tricks to sidestep the toxic effects of these drugs. In fact, a recent study published in the Journal of the American Medical Association suggested that the number of deaths associated with Methicillin-Resistant Staphylococcus aureus (MRSA), exceeds those attributed to AIDS, Parkinson’s disease, emphysema or homicide each year (see JAMA 298:1763-1771). This ever-increasing problem stems from the fact that antibiotic use selects for bacteria with resistance and increases the fraction of total bacteria in the biosphere that are resistant. Bacterial populations have little or no selection pressure against them to change this enrichment of resistant cells in the absence of antibiotics. The goal of our genetic circuit is to program the fitness of antibiotic resistant cells to be lower than that of antibiotic sensitive cells in the presence of non-toxic levels of antibiotic. In this way, we seek to reduce the fraction of antibiotic resistant cells in the biosphere and increase the utility of existing antibiotics.
Details...
Project B: Quorumtaxis
Bacteria have evolved diverse genetic systems to sense their environment as well as respond to their surroundings in an adaptive manner. Of recent intense interest is the discovery that bacteria use pheromones that allow them to sense their own population density, a system known as quorum sensing. Another widely studied system is that of chemotaxis, in which a bacterium is able to sense and adaptively swim towards or away from a chemical agent in the environment. The Rice University iGEM team is attempting to merge these two existing natural systems (quorum sensing and chemotaxis) to produce a novel bacterial phenotype (quorumtaxis) in which the engineered cell will be able to detect and swim towards the quorum pheromone of another ‘target’ cell. This project will demonstrate the ability to produce unique complex behavior in bacteria through the modular integration of existing circuits. In addition, precise control over bacterial movement will greatly increase the complexity of systems synthetic biologists could create. The keystone of the project is the design of a novel chimeric receptor which can sense the target pheromone and then signal flagellar rotation within the cell. This project necessitates a highly interdisciplinary approach: the conceptual design and part construction requires backgrounds in biochemistry and cell biology, protein engineering elements requires experience in biomolecular engineering methods, and computational mathematics will be used to model the quorumtaxis phenotype.
Our iGEM team asks the following question: Can we re-engineer E.coli to recognize and pursue other bacterial species?
Details...
Thanks for visiting our wiki, and be sure to tell us what you think when we see you at the iGEM Jamboree!
2007 Team Roster
- Undergraduate Students
- Tina Chen
- Thomas Segall-Shapiro
- Taylor Stevenson
- David Kim
- Alec Walker
- Arielle Layman
- Graduate Advisors
- Bibhash Mukhopadhyay
- Peter Nguyen
- Christie Peebles
- Jay Raol
- Faculty Advisors
- Beth Beason, PhD.
- George Bennett, PhD.
- Steve Cox, PhD.
- Ken Cox, PhD.
- Ka-yiu San, PhD.
- Joff Silberg, PhD.