Saint Petersburg
From 2007.igem.org
(→Our Project) |
(→About Us) |
||
| Line 9: | Line 9: | ||
**Alexey Shalygin | **Alexey Shalygin | ||
**[https://2007.igem.org/User:Masha Maria Ditina] | **[https://2007.igem.org/User:Masha Maria Ditina] | ||
| - | **[ | + | **[https://2007.igem.org/User:Gena Gennadiy Zakharov] |
*'''Our Instructors:''' | *'''Our Instructors:''' | ||
| - | **[ | + | **[https://2007.igem.org/User:Evgeny Evgeny Zatulovskiy ] |
**Vasiliy Romanov | **Vasiliy Romanov | ||
**[https://2007.igem.org/User:Alexej Alexej Skvortsov] | **[https://2007.igem.org/User:Alexej Alexej Skvortsov] | ||
Revision as of 10:40, 14 August 2007
Saint-Petersburg iGem2007 team
This year our team is participating for the first time in iGem2007 project.
Our team works at the Saint-Petersburg State Polytechnical University in the department of Biophysics.
About Us
- Students:
- Tatiana Moiseeva
- Alexey Shalygin
- Maria Ditina
- Gennadiy Zakharov
- Our Instructors:
- Evgeny Zatulovskiy
- Vasiliy Romanov
- Alexej Skvortsov
Our Project
Copper and copper methabolism
Copper is a unique transition element, that may easily activate dioxygen bond. This makes copper both vitally essential to aerobic species and severely toxic to all organisms at higher concentrations. The toxicity is caused mostly by catalyzing uncontrolled dioxygen conversion to reactive oxygen species via a series of redox reactions (the Fenton cycle). To avoid this problem most organisms evolved copper metabolic systems, so that virtually all copper inside the cell is tightly protein-bound. Still high external concentrations of copper is deadly to many organisms, underlying the well known antifungal and insecticide applications of copper salts in agriculture. Yet copper is not so evident toxin as nickel or cadmium, as many organisms (e.g. higher plants) are resistant to high concentrations of copper.
In our opinion, importance of copper as water pollutant and ecotoxicant is underestimated, probably because its toxic action on natural ecosystems is not so evident.
For humans the main source of excess copper ions is drinking water. The potential copper ion sources are agricultural application, metallic copper corrosion and chemical industry. Adult mammals have a protective system, that controls copper absorption in the intestine and copper excretion through bile. E.g., for adult rats, LD50 oral dose of Cu salts is over 30-fold higher than intraperitoneal LD50 dose (580 vs 15 mg/kg, Safety data for copper (II) chloride). But newborn infants have other type of copper metabolism. Copper absorption and excretion in suckling newborns is not controlled. Copper is provided in exact amounts in protein bound form by milk. So the infant totally relies on delicate copper control of the mammary gland of its mother. This scheme leads to the existence some very peculiar copper disorders, like “toxic milk mice”. The fact means, that copper is a very dangerous element for infants. Excess copper, absorbed by an infant, may inflict serious disorders on the developing brain and cause many delayed problems of “unknown origin”.
The aim of the project
In our project we intend to create a copper biosensor, that would sense copper ions in growing media (based on a water sample). The advantage of biosensor over FAAS and MS techniques is, that it will sense true free copper concentration, available to biological system. It will not react to strongly ligand-bound copper and fine dispersed metallic copper.
To make our sensor more robust we intend to supplement it with a threshold device, that will provide the response on a “all or nothing” basis, when copper level will exceed the critical concentration. So, our project falls into two distinct parts. The first part is a copper-responsive element, that will convert measured copper level to TIPS. The second part is a threshold device with hysteresis (a Schmitt trigger), that is designed as a individual part, so that it may be used with any input.
1. Copper responsive element In the creation of copper responsive element we rely on natural copper sensing systems of E. coli Copper is the essential element for E. coli, so that its intracellular level is strictly regulated. Generally, outside copper concentration exceeds the cooper need of the cell, so that excess copper is excreted. Inside copper level is sensed by CueR protein, that controls the expression of CopA efflux pump. We are not to affect this loopback, as malfunction of it will cause copper poisoning of the cell. We’d rather use another natural copper sensing loopback, that senses outside copper level. It consists of CusR/CusS two component signal system, that controls expression of CusCFBA operon, coding for copper efflu
To solve this problem we need to use the copper-homeostasis system of E.Coli. The most convenient for our purposes protein is CusR, the component of the Copper-Responsive Two-Component System.
2. We perform a theoretical study, to find the best scheme with hysteresis (trigger).
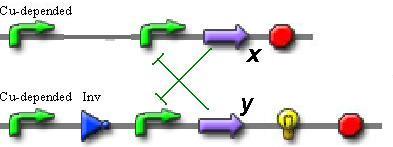
So, if we want to use only proteins without secondary metabolites, the best trigger sheme is the next:
In this scheme we can observe the switching between two repressors (x and y), depending on the transcription level from the copper-depended promoter.
But this scheme is rather difficult and need many constructing operations. So, we decided to think more simple scheme, using the secondary metabolites.