Tokyo/IPTG assay
From 2007.igem.org
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
| + | |||
| + | ==[[Tokyo_Tech|Abstract]] [[Tokyo/Model|Concept & Model]] [[Tokyo/Requirements |Requirements]] [[Tokyo/Genetic circuit|Genetic_circuit]] [[Tokyo/Works|Works]] [[Tokyo/About our team|About_our_team]]== | ||
| + | <br>[[Tokyo/Works|Works top]] 0.[[Tokyo/Works/Hybrid promoter|Hybrid promoter]] 1.[[Tokyo/Works/Formulation |Formulation]] 2.[[Tokyo/Works/Assay |Assay1]] 3.[[Tokyo/Works/Simulation |Simulation]] 4.[[Tokyo/Works/Assay2 |Assay2]] 5.[[Tokyo/Works/Future works |Future works]] | ||
| + | |||
== IPTG assay == | == IPTG assay == | ||
===Purpose: === | ===Purpose: === | ||
Revision as of 21:43, 24 October 2007
Abstract Concept & Model Requirements Genetic_circuit Works About_our_team
Works top 0.Hybrid promoter 1.Formulation 2.Assay1 3.Simulation 4.Assay2 5.Future works
IPTG assay
Purpose:
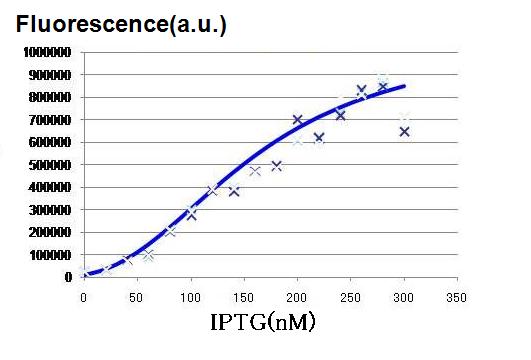
To determine the order of the concentration of IPTG necessary for the activation of our lux-lac hybrid promoter in the LacI producing pTrc99A cells.
The order of the concentration is used for more detailed assay with narrower range of the IPTG concentration.
Samples:
A4 placQI in pTrc99A
A4 ΔP in pTrc99A
Lux-lac hybrid promter + A4 in pBR322
Lux-lac hybrid promter + A4 in pTrc99A
Procedure:
prepare overnight culture for each sample
make fresh culture
take 3 ul of the overinight culture into 3 ml of LB (Amp and/or Kan) in Falcon tubes.
incubate for 2 to 3 hours until the observed OD is around 0.5
add AHL & IPTG solution
[AHL]final (in 3 ml LB culture) = 10 nM
[IPTG]final (in 3 ml LB culture) = 1000, 100, 10, 1, 0.1, 0.01, and 0 mM
incubate for 2 to 3 hours
apply 150 ul of samples into 96-well plaste
FLA measurement