UCSF/Intro
From 2007.igem.org
(Difference between revisions)
| Line 8: | Line 8: | ||
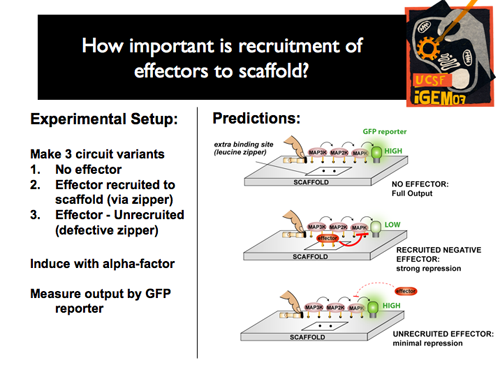
|[[Image:p1intro12.png]]||Experimentally, we wanted not only to compare the range of effects through the effector toolbox, but also to examine the significance of recruitment of each effector the scaffold. So, for each experiment, we created three variants: one with no effector, one with the effector recruited to the scaffold, and one with an effector that was unable to be recruited to the scaffold. We hypothesized that, compared to the full output of the undisturbed pathway, we should see a strong repression with the recruited effector, but only minimal repression without actual recruitment to the scaffold. | |[[Image:p1intro12.png]]||Experimentally, we wanted not only to compare the range of effects through the effector toolbox, but also to examine the significance of recruitment of each effector the scaffold. So, for each experiment, we created three variants: one with no effector, one with the effector recruited to the scaffold, and one with an effector that was unable to be recruited to the scaffold. We hypothesized that, compared to the full output of the undisturbed pathway, we should see a strong repression with the recruited effector, but only minimal repression without actual recruitment to the scaffold. | ||
|} | |} | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <div style="text-align: right;"> <font size=4><font color=firebrick>Move on to see our '''[https://2007.igem.org/UCSF/Results Results!]''' </font></font></div> | ||
Revision as of 20:25, 28 September 2007
Move on to see our Results!