USTC/Repressor Evolution on Plates
From 2007.igem.org
m |
m |
||
| (62 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | == | + | == Wires in Bio-logic Circuit == |
| - | We have been attempting to construct several artificial repressor-operator pairs to serve as the connecting wires of our system, based on the knowledge of LacR and its binding site, and by means of protein design. We decide that they should be newly designed for | + | In electronic circuit, metal conductor such as copper is used as wires widely. But in a cell, most of the things are diffusive, so it is a difficult problem to limit a signal in a specific signal channel. High-specific regulator-operator pairs can be used as "copper wires" in bio-logic circuit. It means, repressor or activator transmit a particular signal from the upstream output port to the downstream input port, without interference between each other, like carrier wave in FM radio. |
| - | # The number of natural regulators well studied is | + | |
| + | |||
| + | === Why to Design Artificial High-Specific Repressor-Operator Pairs === | ||
| + | |||
| + | We have been attempting to construct several artificial high-specific repressor-operator pairs to serve as the connecting wires of our system, based on the knowledge of LacR and its binding site [[USTC/Repressor_Evolution_on_Plates#References|[1, 10]]], and by means of directed evolution and computational protein design. We decide that they should be newly designed for several reasons, | ||
| + | |||
| + | # The number of natural regulators well studied is moderate [[USTC/Repressor_Evolution_on_Plates#References|[11]]]. | ||
# Natural regulators do have some disadvantages. | # Natural regulators do have some disadvantages. | ||
| - | #* For example, it is well known that there are dozens of downstream regulatory sites of CRP in E.Coli, and if we abuse the CRP, some other natural pathways in the host bacterial will probably be interrupted. | + | #* For example, it is well known that there are dozens of downstream regulatory sites of CRP in E.Coli, and if we abuse the CRP, some other natural pathways in the host bacterial will probably be interrupted [[USTC/Repressor_Evolution_on_Plates#References|[2]]]. |
| - | # There may be several unknown sites to be bound with the selected native activators, and we might get unexpected results in such situations. | + | #* There may be several unknown sites to be bound with the selected native activators, and we might get unexpected results in such situations. |
| + | # This artificial repressor family hold the same property for our cis-acting logic promoters, such as dimerization ad tetramerization [[USTC/Repressor_Evolution_on_Plates#References|[1]]]. | ||
| - | + | [[Image:USTC_allen1.jpg|thumb|center|500px|'''Figure 1''' Comics: All we need are the specific artificial 'repressor fish' that can definitely bite the specific 'operator hook' exclusively]] | |
| - | |||
| - | + | == Start: Lac repressor == | |
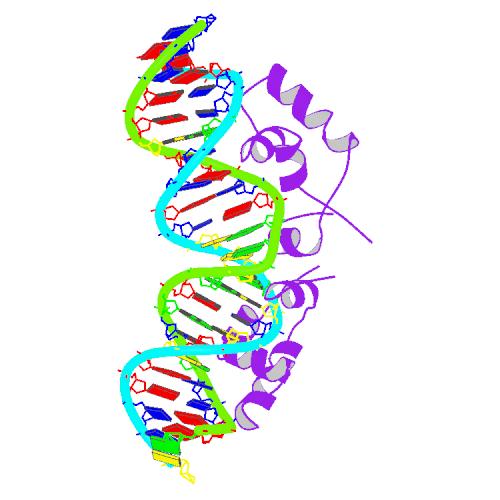
| - | + | [[Image:USTC_1l1m_bio_r_500.jpg|thumb|'''Figure 2''' Solution structure of a dimer of Lac repressor DNA-binding domain complexed to its natrual operator O1 ([http://www.rcsb.org/pdb/explore.do?structureId=1L1M From RCSB])]] | |
| - | The lac repressor | + | The lac repressor in Lac operon is a well-studied transcriptional factor involved in the metabolism of lactose in bacteria. It has three distinct regions : |
| - | * a core region | + | * a headpiece which recognize DNA and joins two monomers to form a dimer at the same time |
| - | * a tetramerization region | + | * a core region which binds allolactose and IPTG |
| - | + | * a tetramerization region which joins four monomers in an alpha-helix bundle) | |
| - | The | + | The DNA binding region of Lac repressor consists of a helix-turn-helix structural motif and has been well studied as a model structure of transcription factor in helix-turn-helix family[[USTC/Repressor_Evolution_on_Plates#References|[1,3]]]. |
| - | |||
== Selection of operator sequence == | == Selection of operator sequence == | ||
| - | By means of bioinformatics we can select a DNA sequence that has never appeared in the genome of E.Coli, and let the regulator bind to the sequence with quite high specificity | + | By means of bioinformatics we can select a DNA sequence that has never appeared in the genome of E.Coli, and let the regulator bind to the sequence with quite high specificity and high affinity [[USTC/Repressor_Evolution_on_Plates#References|[6]]]. Therefore, we will not have to worry about the regulator’s interrupting the normal functioning of the host genome (A tiny exception: the host strain should not contain <i>lacI</i> and lac promoter, such as [http://openwetware.org/wiki/E._coli_genotypes#TOP10_.28Invitrogen.29 Top10]). |
| + | |||
| + | O11 aattgtgagcgctcacaatt | ||
| + | O22 aattgtaagcgcttacaatt | ||
| + | O33 aattgtaaacgtttacaatt | ||
| + | O44 aattgtgaacgttcacaatt | ||
| + | O55 aattttgagcgctcaaaatt | ||
| + | O66 aattatgagcgctcataatt | ||
| + | O77 gacgactgtatacagtcgtc | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | == Directed Repressor Evolution == | ||
| + | |||
| + | === Targeted Mutagenesis === | ||
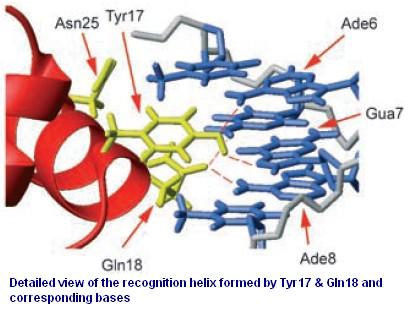
| + | [[Image:USTC_allen2.jpg|thumb|right|300px|'''Figure 3''' The recognition region of Lac repressor of which the amino acid residues we will try to modify. This figure comes from 'Roberto Kopke Salinas, etc. ''ChemBioChem 2005, 6, 1628 – 1637'']] | ||
| + | |||
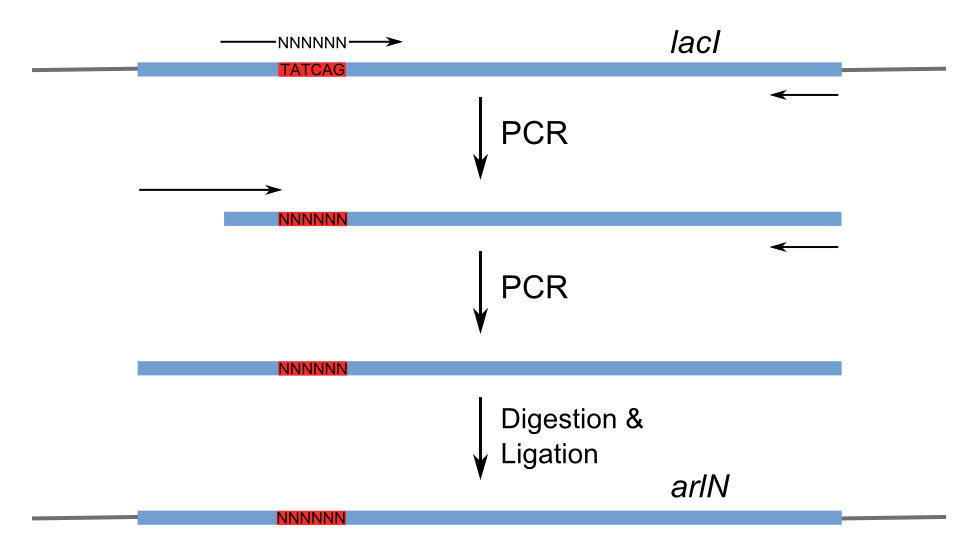
| + | Figure 3 shows the recognition region of which the amino acid residues we will try to modify. With saturation mutagenesis [[USTC/Repressor_Evolution_on_Plates#References|[4, 5]]], it is possible to create a library of mutants containing all possible mutations at these positions. Because the targeted sites are near to the beginning of the <i>lacI</i> gene yet beyond the range of one primer region, two steps of PCR are carried out to generate the random repressor family shown in Figure 4. Then the PCR production is purified and digested, loaded into the repressor-production plasmid to take the repression assay. | ||
| + | |||
| + | [[Image:USTC_RepressorMutagenesis.png|thumb|left|350px|'''Figure 4''' Random repressor family generation by PCR.]] | ||
| + | |||
| + | <BR clear="both"> | ||
| + | |||
| + | === Repression Assay === | ||
| + | |||
| + | With the help of pUC-repressor and pZS*-reporter, the two plasmids shown in Figure 5, we will be able to accomplish the repression assay. | ||
| + | |||
| + | The first plasmid is in charge of repressor production, that is, to express the repressor constitutively. The second plasmid contains two reporter genes, <i>lacZ</i> (alpha fragment) and <i>gfp-AAV</i>, respectively, and an upstream promoter that reflects the repression effect in the form of binding intensity. Once the repression exists, the promoter will lose its activity. Consequently, neither of the downstream lacZ or GFP-AAV reporter gene will work. By reading the blue/white colonies on a plate with naked eyes and the fluorescence intensity of GFP with a fluorescence microscope, we can finally get to know the repression effect, and can ratiocinate from it the binding affinity of the repressor-operator pair. | ||
| + | |||
| + | [[Image:ustc_repressor_assay.png|thumb|center|600px|'''Figure 5''' (a) Left: Plasmid pUC-repressor, a repressor-production plasmid. Right: Plasmid pZS*-reporter, a repression-reporter plasmid. If the repressor produced by pUC-repressor is able to repress the promoter on pZS*-reporter, neither the activity of beta-galactosidase nor the expression of GFP will be observed. (b) Scheme of the promoter on the pZS*-reporter. There are two repressor binding sites around the RNA polymerase binding site. If the designed repressor is able to bind the designed operator, the activity of the promoter will be repressed.]] | ||
| + | |||
| + | === Screening === | ||
| + | |||
| + | First, pUC-repressor plasmid containing random repressor family members are transformed into Top10/pZS*-PO_target-lacZa. White colonies, which shows that repressors there can repress the target promoter, are select from Blue/White Screening on top agar Luria-Bertani broth. The repression should be re-tested to eliminate the false positive samples by X-gal assay and PCR checking. The survivals will be quantificationally measured in ONPG assay or fluorescent assay. | ||
| + | |||
| + | [[Image:USTC_repressor_screening.jpg|thumb|center|600px|'''Figure 6''' (a) One of the Blue/White screening plates. The red-marked colony are a target. (b) One of the plates in the repression assay. The blue or light blue colonies show the repressor expressed in the strains cannot or can weakly binding to the specified operator sequence, and in contrast, the repressor produced in the white colonies can repress the specified promoter. (c) PCR checking for promoter and lacZ alpha fragment.]] | ||
| + | |||
| + | ===Quantitive Assay(Cross Repression Test)=== | ||
| + | We selected 7 repressor-promoter pair candidates from Blue/White Screening results above for quantitive assay of specificity and affinity. In addition, 2 existed represor-promoter pairs are added to this work as new candidates. Then, each repressor-expression plasmid is transform to each Top10 competent cell with specific target promoters. Eventually, each expression quantity of LacZ alpha or GFP is measured by ONPG assay(LacZ) or fluorescent assay(GFP).The process are shown as below. | ||
| + | |||
| + | [[Image:USTC_ crossrepressiontest.jpg|center|600px]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | ---- | ||
| + | == Results == | ||
| + | |||
| + | The consequent data of reporter's expression is, by formula, converted to the Repression Value(R.V.) representing the repression intensity of each repressor-promoter pair. From the formula below we can see higher value of R.V. represents the higher expression of reporter gene and indicates a lower repression while the reverse represents a high repression. The sets of R.V. is depicted on the scheme below which have been transformed to corresponding Repression Matrix-more visual that the coordinate scheme. And the Orthogonal Repression Matrix birthing from Repression Matrix can be used to acquire specific repressor-promoter pairs with high specificity. | ||
| + | |||
| + | [[Image:USTC_rv.jpg|500px|center]] | ||
| + | |||
| + | |||
| + | <!-- | ||
| + | === Repression Scheme === | ||
| + | |||
| + | The directed results are charted on one coordinate scheme. | ||
| + | |||
| + | [[Image:USTC_RepressionScheme.jpg|thumb|center|550px|'''Figure 7''' This scheme indicates the cross repression test of our repressor and promoter candidates. The vertical axis tell us the Repression Value(R.V.) of each repressor-promoter pairs by plots, in which the higher R.V.shows the higher expression of reporter protein(even exceed the condition without repressors)-the low repression. The horizontal axis represents the different repressors, and the colors represent different promoters]] | ||
| + | --> | ||
| - | + | === Repression Matrix === | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Performances of our wires are shown as so-called "Repression Matrix", an array of R.V. with variant combinations of artificial repressors and operators. A "Repression Matrix" taken from the literature [[USTC/Repressor_Evolution_on_Plates#References|[7,8,9]]] and uniformed is also plotted in [[USTC/RepressionMatrixFromLiterature|this page]]. | |
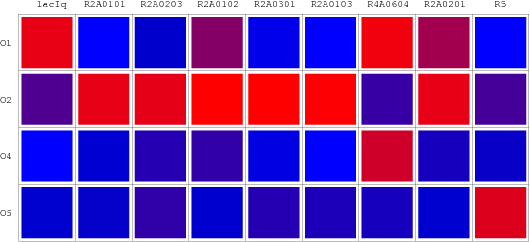
| - | [[Image: | + | [[Image:USTC_RepressionMatrix.png|thumb|center|500px|'''Figure 8''' Repression Metrix(RM):The repression matrix reveals the binding affinity of different repressor candidates with various specific promoters by different colors. The deeper the red will be, the higher the repression will appear. While the lighter the blue is the weaker the repression will show.]] |
| - | + | === Orthologal Repression Matrix === | |
| - | + | [[Image:USTC_OrthologalRepressionMatrix.png|thumb|rught|200px|'''Figure 9''' Orthologal Repression Metrix(ORM)-One of the ORM comes from above RM:The red diagonal of this matrix indicates that each target repressor can only firmly bind to its specific target promoter and has weak or even no binding to other operator sequences.]] | |
| + | Several 'wires' without interference are selected based on that repressors can only specifically bind to the right promoter without strong repressed interaction with other promoters which have their own special repressors. Thereby, the red color plot in Repression Matrix should be placed on the right site by interchange of columns so that each row and each column in the Matrix can have only one red plot. The other repressor candidate columns which cannot pass muster will be deleted from the RM. Eventually, a 3x3 Orthogonal Repression Matrix for [[USTC/Demonstration|the demonstration system]] shown in Figure 9, which looks like an orthogonal matrix in mathematics, contains single red plot in each column & each row, reflecting the specific repression. | ||
| + | ---- | ||
| + | == References == | ||
| + | 1. Lewis, M. (2005), 'The lac repressor.', <i>C R Biol 328</i> (6), 521--548. | ||
| + | 2. Lac Operon, http://en.wikipedia.org/wiki/Lac_Operon | ||
| + | 3. Kalodimos, C. G.; Bonvin, A. M. J. J.; Salinas, R. K.; Wechselberger, R.; Boelens, R. & Kaptein, R. (2002), 'Plasticity in protein-DNA recognition: lac repressor interacts with its natural operator 01 through alternative conformations of its DNA-binding domain.', <i>EMBO J</i> 21(12), 2866--2876. | ||
| + | 4. Arnold, Frances H. and Georgiou, George. ' Directed Evolution Library Creation, Methods and Protocols', <i>Methods in Molecular Biology<i>, Humana Press, Volume 231, 2003 | ||
| + | 5. Arnold, Frances H. and Georgiou, George. ' Directed Evolution Library Creation, Screening and Selection Methods', <i>Methods in Molecular Biology</i>, Humana Press, Volume 230, 2003 | ||
| - | + | 6. Sadler, J. R.; Sasmor, H. & Betz, J. L. (1983), 'A perfectly symmetric lac operator binds the lac repressor very tightly.', <i>PNAS</i> 80(22), 6785--6789. | |
| - | + | 7. Lehming, N.; Sartorius, J.; Niemöller, M.; Genenger, G.; v Wilcken-Bergmann, B. & Müller-Hill, B. (1987), 'The interaction of the recognition helix of lac repressor with lac operator.', <i>EMBO J</i> 6(10), 3145--3153. | |
| - | + | 8. Lehming, N.; Sartorius, J.; Oehler, S.; von Wilcken-Bergmann, B. & Müller-Hill, B. Recognition helices of lac and lambda repressor are oriented in opposite directions and recognize similar DNA sequences. <i>PNAS</i>, 1988, 85, 7947-7951 | |
| - | + | 9. Sartorius, J.; Lehming, N.; Kisters, B.; von Wilcken-Bergmann, B. & Müller-Hill, B. (1989), 'lac repressor mutants with double or triple exchanges in the recognition helix bind specifically to lac operator variants with multiple exchanges.', <i>EMBO J</i> 8(4), 1265--1270. | |
| - | + | 10. Salinas, R. K.; Folkers, G. E.; Bonvin, A. M. J. J.; Das, D.; Boelens, R. & Kaptein, R. Altered specificity in DNA binding by the lac repressor: a mutant lac headpiece that mimics the gal repressor.' <i>Chembiochem</i>, 2005, 6, 1628-1637 | |
| - | + | ||
| - | + | 11. Müller-Hill, B. 'Some repressors of bacterial transcription.' <i>Curr Opin Microbiol</i>, 1998, 1, 145-151 | |
Latest revision as of 01:37, 27 October 2007
Contents |
Wires in Bio-logic Circuit
In electronic circuit, metal conductor such as copper is used as wires widely. But in a cell, most of the things are diffusive, so it is a difficult problem to limit a signal in a specific signal channel. High-specific regulator-operator pairs can be used as "copper wires" in bio-logic circuit. It means, repressor or activator transmit a particular signal from the upstream output port to the downstream input port, without interference between each other, like carrier wave in FM radio.
Why to Design Artificial High-Specific Repressor-Operator Pairs
We have been attempting to construct several artificial high-specific repressor-operator pairs to serve as the connecting wires of our system, based on the knowledge of LacR and its binding site [1, 10], and by means of directed evolution and computational protein design. We decide that they should be newly designed for several reasons,
- The number of natural regulators well studied is moderate [11].
- Natural regulators do have some disadvantages.
- For example, it is well known that there are dozens of downstream regulatory sites of CRP in E.Coli, and if we abuse the CRP, some other natural pathways in the host bacterial will probably be interrupted [2].
- There may be several unknown sites to be bound with the selected native activators, and we might get unexpected results in such situations.
- This artificial repressor family hold the same property for our cis-acting logic promoters, such as dimerization ad tetramerization [1].
Start: Lac repressor
The lac repressor in Lac operon is a well-studied transcriptional factor involved in the metabolism of lactose in bacteria. It has three distinct regions :
- a headpiece which recognize DNA and joins two monomers to form a dimer at the same time
- a core region which binds allolactose and IPTG
- a tetramerization region which joins four monomers in an alpha-helix bundle)
The DNA binding region of Lac repressor consists of a helix-turn-helix structural motif and has been well studied as a model structure of transcription factor in helix-turn-helix family[1,3].
Selection of operator sequence
By means of bioinformatics we can select a DNA sequence that has never appeared in the genome of E.Coli, and let the regulator bind to the sequence with quite high specificity and high affinity [6]. Therefore, we will not have to worry about the regulator’s interrupting the normal functioning of the host genome (A tiny exception: the host strain should not contain lacI and lac promoter, such as [http://openwetware.org/wiki/E._coli_genotypes#TOP10_.28Invitrogen.29 Top10]).
O11 aattgtgagcgctcacaatt O22 aattgtaagcgcttacaatt O33 aattgtaaacgtttacaatt O44 aattgtgaacgttcacaatt O55 aattttgagcgctcaaaatt O66 aattatgagcgctcataatt O77 gacgactgtatacagtcgtc
Directed Repressor Evolution
Targeted Mutagenesis
Figure 3 shows the recognition region of which the amino acid residues we will try to modify. With saturation mutagenesis [4, 5], it is possible to create a library of mutants containing all possible mutations at these positions. Because the targeted sites are near to the beginning of the lacI gene yet beyond the range of one primer region, two steps of PCR are carried out to generate the random repressor family shown in Figure 4. Then the PCR production is purified and digested, loaded into the repressor-production plasmid to take the repression assay.
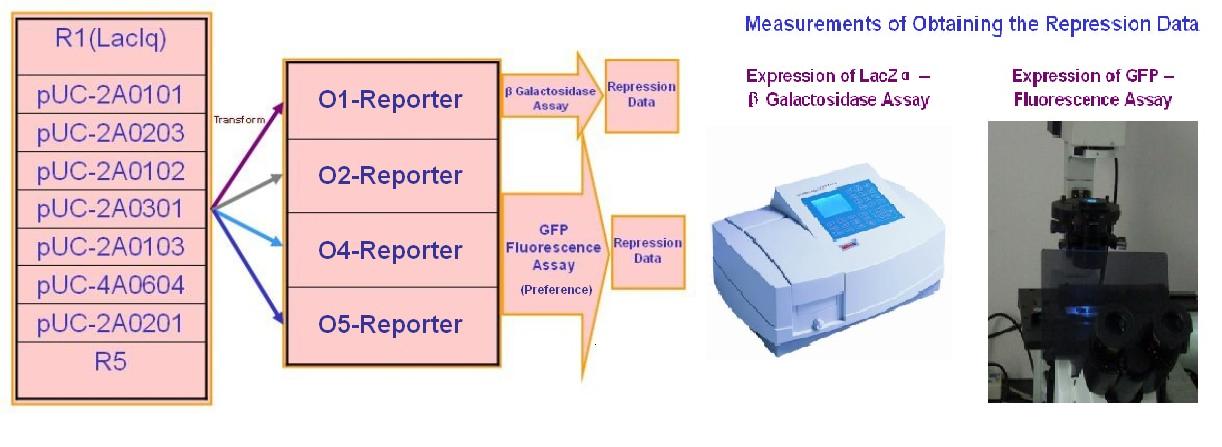
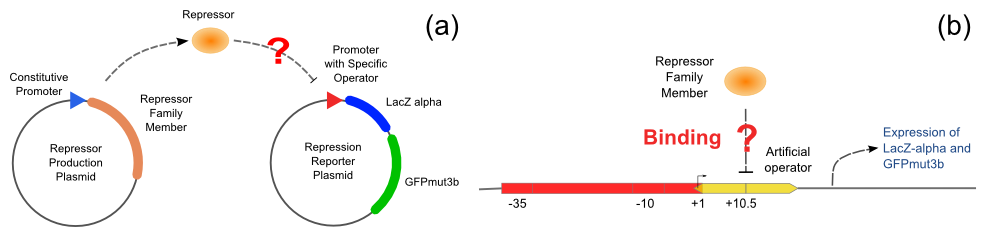
Repression Assay
With the help of pUC-repressor and pZS*-reporter, the two plasmids shown in Figure 5, we will be able to accomplish the repression assay.
The first plasmid is in charge of repressor production, that is, to express the repressor constitutively. The second plasmid contains two reporter genes, lacZ (alpha fragment) and gfp-AAV, respectively, and an upstream promoter that reflects the repression effect in the form of binding intensity. Once the repression exists, the promoter will lose its activity. Consequently, neither of the downstream lacZ or GFP-AAV reporter gene will work. By reading the blue/white colonies on a plate with naked eyes and the fluorescence intensity of GFP with a fluorescence microscope, we can finally get to know the repression effect, and can ratiocinate from it the binding affinity of the repressor-operator pair.
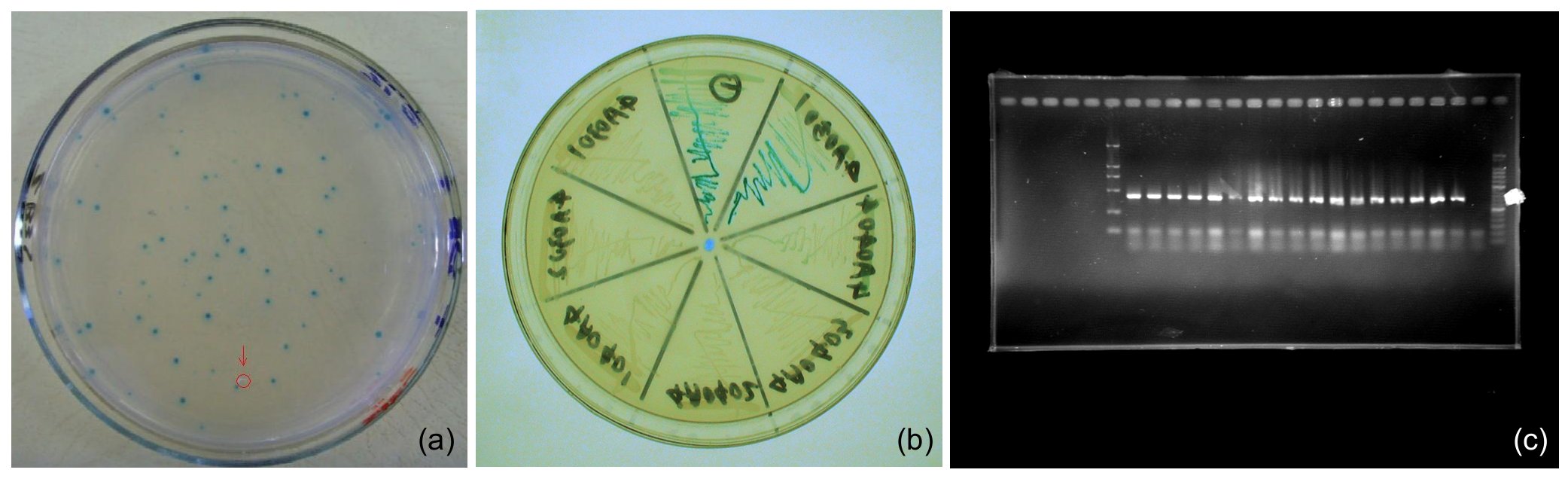
Screening
First, pUC-repressor plasmid containing random repressor family members are transformed into Top10/pZS*-PO_target-lacZa. White colonies, which shows that repressors there can repress the target promoter, are select from Blue/White Screening on top agar Luria-Bertani broth. The repression should be re-tested to eliminate the false positive samples by X-gal assay and PCR checking. The survivals will be quantificationally measured in ONPG assay or fluorescent assay.
Quantitive Assay(Cross Repression Test)
We selected 7 repressor-promoter pair candidates from Blue/White Screening results above for quantitive assay of specificity and affinity. In addition, 2 existed represor-promoter pairs are added to this work as new candidates. Then, each repressor-expression plasmid is transform to each Top10 competent cell with specific target promoters. Eventually, each expression quantity of LacZ alpha or GFP is measured by ONPG assay(LacZ) or fluorescent assay(GFP).The process are shown as below.
Results
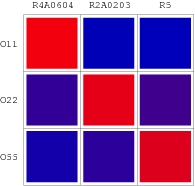
The consequent data of reporter's expression is, by formula, converted to the Repression Value(R.V.) representing the repression intensity of each repressor-promoter pair. From the formula below we can see higher value of R.V. represents the higher expression of reporter gene and indicates a lower repression while the reverse represents a high repression. The sets of R.V. is depicted on the scheme below which have been transformed to corresponding Repression Matrix-more visual that the coordinate scheme. And the Orthogonal Repression Matrix birthing from Repression Matrix can be used to acquire specific repressor-promoter pairs with high specificity.
Repression Matrix
Performances of our wires are shown as so-called "Repression Matrix", an array of R.V. with variant combinations of artificial repressors and operators. A "Repression Matrix" taken from the literature [7,8,9] and uniformed is also plotted in this page.
Orthologal Repression Matrix
Several 'wires' without interference are selected based on that repressors can only specifically bind to the right promoter without strong repressed interaction with other promoters which have their own special repressors. Thereby, the red color plot in Repression Matrix should be placed on the right site by interchange of columns so that each row and each column in the Matrix can have only one red plot. The other repressor candidate columns which cannot pass muster will be deleted from the RM. Eventually, a 3x3 Orthogonal Repression Matrix for the demonstration system shown in Figure 9, which looks like an orthogonal matrix in mathematics, contains single red plot in each column & each row, reflecting the specific repression.
References
1. Lewis, M. (2005), 'The lac repressor.', C R Biol 328 (6), 521--548.
2. Lac Operon, http://en.wikipedia.org/wiki/Lac_Operon
3. Kalodimos, C. G.; Bonvin, A. M. J. J.; Salinas, R. K.; Wechselberger, R.; Boelens, R. & Kaptein, R. (2002), 'Plasticity in protein-DNA recognition: lac repressor interacts with its natural operator 01 through alternative conformations of its DNA-binding domain.', EMBO J 21(12), 2866--2876.
4. Arnold, Frances H. and Georgiou, George. ' Directed Evolution Library Creation, Methods and Protocols', Methods in Molecular Biology<i>, Humana Press, Volume 231, 2003
5. Arnold, Frances H. and Georgiou, George. ' Directed Evolution Library Creation, Screening and Selection Methods', <i>Methods in Molecular Biology, Humana Press, Volume 230, 2003
6. Sadler, J. R.; Sasmor, H. & Betz, J. L. (1983), 'A perfectly symmetric lac operator binds the lac repressor very tightly.', PNAS 80(22), 6785--6789.
7. Lehming, N.; Sartorius, J.; Niemöller, M.; Genenger, G.; v Wilcken-Bergmann, B. & Müller-Hill, B. (1987), 'The interaction of the recognition helix of lac repressor with lac operator.', EMBO J 6(10), 3145--3153.
8. Lehming, N.; Sartorius, J.; Oehler, S.; von Wilcken-Bergmann, B. & Müller-Hill, B. Recognition helices of lac and lambda repressor are oriented in opposite directions and recognize similar DNA sequences. PNAS, 1988, 85, 7947-7951
9. Sartorius, J.; Lehming, N.; Kisters, B.; von Wilcken-Bergmann, B. & Müller-Hill, B. (1989), 'lac repressor mutants with double or triple exchanges in the recognition helix bind specifically to lac operator variants with multiple exchanges.', EMBO J 8(4), 1265--1270.
10. Salinas, R. K.; Folkers, G. E.; Bonvin, A. M. J. J.; Das, D.; Boelens, R. & Kaptein, R. Altered specificity in DNA binding by the lac repressor: a mutant lac headpiece that mimics the gal repressor.' Chembiochem, 2005, 6, 1628-1637
11. Müller-Hill, B. 'Some repressors of bacterial transcription.' Curr Opin Microbiol, 1998, 1, 145-151