Valencia/August
From 2007.igem.org
Work Progress
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
← July
1/08/07
We're not obtaining anything, our bacterias don´t grow...While the experimental team works at the laboratory, the theoretical team is working in the model and the simulation.
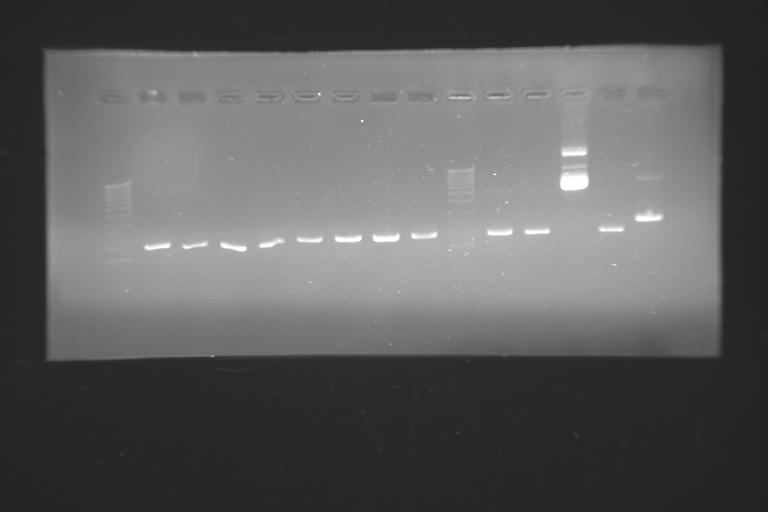
It's wonderful how the lab can manage to teach humility even in the most unexpected way. Not even we have problems transforming bacterias, but the few minipreps we managed to do have showed up no clear band on a gel... May it be that the electrophoresis has made clear some blurring facts that were quite confusing...?? is the problem on the contaminations or on the minipreps?? how come, if it's the case, that a plain DH5α cell grows on a media full of ampicillin?? any clue anyone??
2/08/07
A good new: we have obtained a transformation!! The bacterias have grown with our plasmid (RBS with lacI).
3/08/07
We glycerinate two colonies of weak RBS-LacI.
Minipreps of the two culture. using, now, 50 μl of elution buffer.
Electrophoresis in agarose gel --> now we really know that we have plasmids.
6/08/07
We inoculate the rest of BioBricks needed to build the comparator
As controls, we inoculate a control plasmid too and plain LB
7/08/07
We have another colony of a BioBrick (strong RBS-LacI), as well as the control plasmid
The inoculated LB had no ampicillin-resistent bacteria We transform a commercial pUC, pTet, pLac and the tet repressor with a strong RBS and a weak one
8/08/07
Only the control pUC-18 has grown... We keep transforming the rest of the parts... we're waiting for the commercial cells...
9/08/07
Not a single colony... but... The commercial XL1-blue have just arrived and we have not waited long to transform on it the rest that wasn't yet transformed
We've seen that we have some lines on the gels that should not be there... we're thinking on some contaminating DNA: the lines are heavier than our plasmid and are present in all the colonies we've miniprepped. we're trying other procedures in orther to spot where the contamination could be present. this should not be a problem for the ligations if it's a miniprep problem, but could be an issue if the problems relates to the cell strain (that's why we're transforming right now with our XL1-blue and the commercial ones)
so, by now, we have two main problems: the low transformation efficiency and the alien bands on the gels.
10/08/07
yes... the transformations have worked smoothly, and we now have all the parts we lacked... this night we will have a toast on it! on monday we will start with the ligations... looks like we're on the mood now...
we still have the alien bands on the gel, but we have all the weekend to think about that problem...
the electrophoresis of the minipreps lead us to think that we have lots of false positives: clones that grow on ampicillin petri diches without having our plasmid... the screening should be done with care...
13/08/07
we have identified some colonies of strong RBS with repressor tetR that have the plasmid of the correct size and do not have the alien band on the gel.
we already had some colonies of strong RBS with repressor lacI, so that means that if this afternoon we identifiy some clones of some promoter that are good, we can move on to the ligations... looks like we're going forward and upward...
as for the afternoon, the promoters of the plasmids have grown well, we do have pTetR and pLacI...
tomorrow, we'll go ligating... (there's a funny false friend in spanish with that expression, that we will explain some other day...)
14, 15 & 16/08/07
During this last tree day we were trying to get a ligation, but weren’t lucky.
During the observation from the gel from the first digestion we saw how, just from our eyes, the EtB disappeared and within it the bands whit our plasmid... really weird !!!
on the 15th, the second try wasn’t better. the gel wasn’t well polymerized, so the bands were more spots within all the gel.
So, the third try was the good one and it allowed us to cut the gel and make the ligation on 16th… we will see tomorrow if it works….
17/08/07
Wow, wow, wow, we are very happy… we have colonies… and from both ligations! This morning we started another transformation using another buffer.
the colonies have been inoculated from yesterday transformations... let's hope the screening on the gel will tell us if they are what they should...
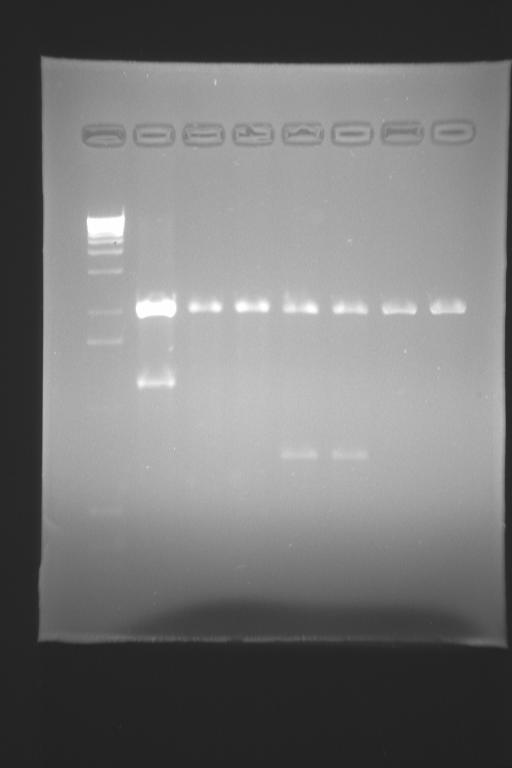
the electrophoresis has shown that we DO have clones that are pLac-tetR and pTet-LacI... next week we'll go forward...
20/08/07
today we have transformed RBS-CFP-T and RBS-YFP-T
we hope to have some colonies tomorrow
21/08/07
we have some colonies!
we are inoculating them and we hope to miniprep them today
hu ho... nothing grew... we are preparing the inoculating them and let's hope that o/n they grow better...
22/08/07
the o/n has grown ok... we think that yesterday it did not grow because we let them too few hours...
- we miniprepped YFP only, because the cultures of CFP did not grow well enough.
- we digested YFP and pTetR-LacI (from a previous miniprep, from the ligated clones from the 17)
- we inoculate CFP so to have the fourth biobrick and go on on the assembly line.
23/08/07
today we have undergone the whole molecular biology process. from the digestion to the tranformation... it's been long...
- we miniprepped the o/n culture
- we digested the CFP biobrick and pLacI-TetR (from a previous miniprep we had from day 17)
- we purified CFP, pLac-TetR, YFP and pTetR-LacI
- on the afternoon, we ligated pTetR-LacI-YFP and pLacI-tetR-CFP
- we transformed it on our Xl1-blue cells... we finish it at 1 a.m.
24/08/07
the Petri dishes are still empty at 10 in the morning... let's hope they have some colonies on the aftenoon...
in the afternoon there are no colonies in either Petri dishes...
we'll come on monday and start again digesting and purifying...
27/08/07
yesterday evening Raul came and inoculated the BioBricks we want to assemble: pLacI-tetR, pTetR-LacI, YFP and CFP.
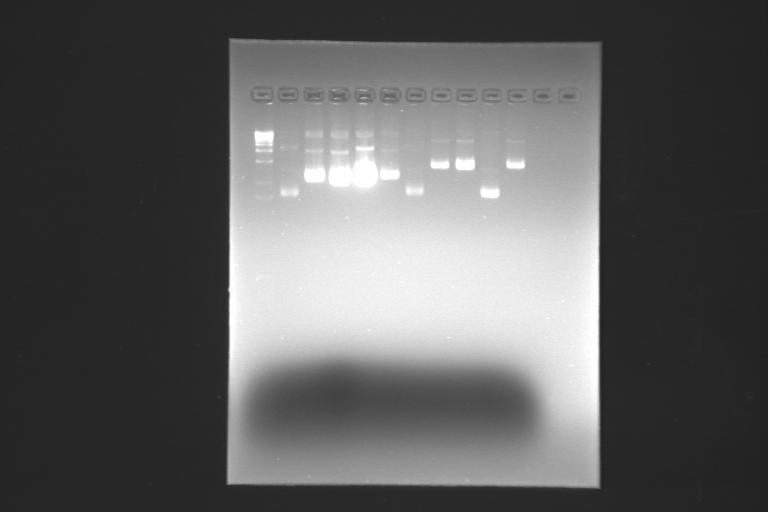
we digest each BioBrick and purify it, but on the purification we loose a lot of DNA yield...
28/08/07
in order to have some more DNA yield at the end of the purification, we decide to do more minipreps and then purify them, joinig and concentrating minipreps that come from the same clone.
we digest and purify the different BioBricks...
the 'disappearing Ethidium Bromide monster' has again shown up and screwed up one of our purification gels...
29/08/07
looks like the DNA yield is still low... we continue digesting and purifying...
30/08/07
using the SpeedVac we are able to concentrate the vectors and inserts so that the resulting ligation mix is 20uL maximum. (i.e. because one of the factors that are said to affect the transformation efficiency of a ligation is the relatively high volume of the ligation mix on the transformation reaction mix)
we, finally, do the ligation of our BioBricks...
transform...
and cross fingers...
31/08/07
there are almost twenty colonies in total... but only six of them grow on the liquid culture with ampicillin... hu ho... contamination danger!
we do the minipreps and keep the plasmids on the fridge...