Virginia/Projects/1
From 2007.igem.org
| HOME | PROJECT INTRO | APPROACH | PROCEDURES | RESULTS | BIOBRICKS | eNOTEBOOK | WEBSITE |
Harvesting Biomass and Light to Power Butanol Biosynthesis
Approach
We have designed a metabolic system capable of cellulose degradation and light metabolism in order to power the biosynthesis of butanol fuel. This hybrid molecular engine is built from standard biological parts and may be used in future designs in order to drive forward cellular chemistry.
The coming years are going to require us to revamp our notions of a fuel economy. Our team hopes to show how synthetic biology can aid in tackling real-world problems not only to aid in the development of new fuel technologies, but also to help support the synthetic biology community by showing it's utility.
Background, Motivation, and References
Why Butanol?
As energy demands increase, the need for alternative fuel sources increases dramatically. US market size for butanol: 370 million gallons per year at a price of about $3.75 per gallon. That’s $1.4 billion.
What is it used for?
Chemical and textile solvent, organic synthesis, chemical intermediate, paint thinner, base of perfumes, and, most importantly, as a biofuel.
As a Biofuel
Butanol biofuel can be used in cars without making engine modifications. It produces more power than ethanol and almost as much power as gasoline.
Butanol better tolerates water contamination and is less corrosive than ethanol and more suitable for distribution through existing pipelines for gasoline.
Why isn’t it more widely spread?
Historically low yields and low concentrations of biobutanol when compared to bioethanol have prevented industry from having stronger interest.
Product tolerance is the main issue. Butanol-producing bacteria (Clostridia acetobutylicum) become limited in growth at approximately 2.5% concentration. Isolating the product at this concentration is not economical.
In the 1950s butanol production shifted from fermentation to being petrochemically-derived. This method continues to be the most popular today.
There are developments in biobutanol production, however. Recently BP and Dupont announced the conversion of an ethanol fermentation facility in the UK to a dedicated biobutanol plant. Biobutanol from this plant will be introduced in 2007.
References:
Our Project
Our goal is to isolate the pathway of butanol production existing in various organisms and engineering the metabolic pathway of E.Coli to produce butanol. Butanol limits bacterial growth by degrades cellular membranes, so the first step is to convey butanol tolerance to E.Coli. This will be accomplished via the use of tolerance genes from other bacterial species.
Next, we will transform the cells with the necessary enzymes for butanol production. These are explained in detail below. By growing the cells in anaerobic conditions and analyzing their product, we hope to tweak the pathway to produce maximum amounts of butanol.
One approach to this is to vary the energy sources the bacteria can use. By inserting genes coding for cellulase, we hope to give our cells the ability to use cellulose as an energy source. Agricultural waste would then become the feed for our strains. Additionally, the use of proteorhodopsin to supplement ATP production is planned. Proteorhodopsin allow the cells to harness light energy independent of oxygen in the environment and drive cellular metabolism.
Our final goal is to design a system that will allow E.Coli to be tolerant to butanol, produce butanol, and do so by exploiting various energy sources to increase efficiency and large-scale feasibility.
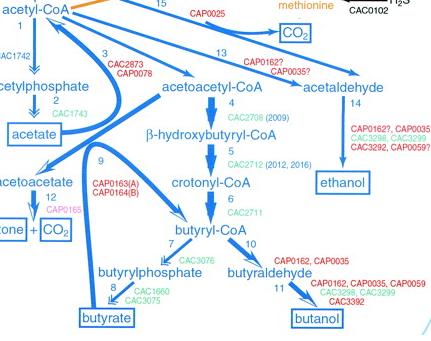
Biobrick and pathway design
Image source: http://jb.asm.org/cgi/content/full/183/16/4823/F5
This is the pathway we are incorporating into E.Coli.
Clostridium acetbutylicum has been known to produce butanol anaerobically in nature. For our project we will be utilizing two butanol sythesis pathways available to us from its genome. In addition to this, we plan to provide a carbon source for butanol biosythesis by importing a cellulase gene from Saccaruphagus degradans, and we plan to facilitate the proton gradient required for ATP sythesis through the use of a proteorhodopsin system.
The diagram below depicts the complete system that we plan to create. The added pathways are: 3, 4, 5, 6, 9, 10, 11. Excluded from this image is our plans for the cellulose digestion pathway, which essentially feed into the production of pyruvate.
The specific proteins we need the cells to produce for this pathway are:
- Specific cellulase
- butanol tolerance genes
- thiolase
- beta-hydroxybutyryl-CoA dehydrogenase
- crotonase
- butyryl coa dehydrogenase
- AAD/AAD2
- alcohol dehydrogenase
- AOTC/AOTD
We have designed the following biobricks:
Need this info.