NYMU Taipei/My hot teams/Engineered cellular system for regulated insulin serection
From 2007.igem.org
Contents |
Brain Storming
Introduction and background
-
Diabetes mellitus
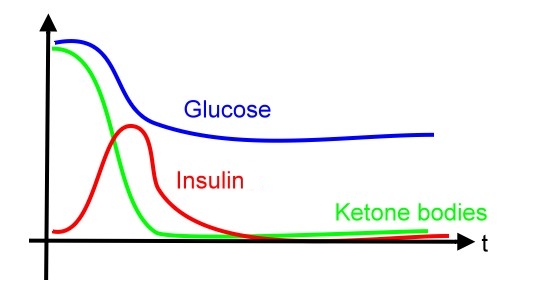
Over 7 percent of adults in the United States (US) are known to have diabetes mellitus. However, because of the associated microvascular and macrovascular disease, diabetes accounts for almost 14 percent of US health care expenditures, at least one-half of which are related to complications such as myocardial infarction, stroke, end-stage renal disease, retinopathy, and foot ulcers. Compared to well-known type 2 diabetes mellitus, manifested with different degrees of insulietobodiesn resistance, type 1 diabetes mellitus, one of the most common chronic diseases in childhood, is caused by insulin deficiency following destruction of the insulin-producing pancreatic beta cells. Data from the Center of Disease Control (CDC) show that more than 13,000 children and adolescents (19 years of age) are diagnosed with type 1 disease each year with a prevalence of 1.7 per 1000. Control of blood sugar and prevention of severe complications, such;as diabetic ketoacidosis (DKA), are important issues nowadays.
-
diabetes and blood glucose levels
In diabetic patient, the glucose level will be out of control after each meal when comparing with the normal person due to impaired insulin production.
-
Ketone bodies
Ketone bodies are produced when fatty acids undergo oxidation. It includes acetone, acetoacetate and D-beta-hydroxybutyrate. Acetone is exhaled and produced less than other two compounds. Acetoacetate and D-beta-hydroxybutyrate are transported to extrahepatic tissues, reconverted to acetyl-CoA and undergo TCA cycle(Krebs cycle) to provide energy.
-
Formation of ketone bodies
Ketone bodies form when carbodyhrate is scarece and cells use fatty acid as the enery source. Formation occurs in the mitochondrial matrix of liver cells. Two molecules of acetyl-CoA first be condensed to one acetoacetyl-CoA by thiolase. Acetoacetyl-CoA is further condensed with one more acetyl-CoA to form HMG-CoA, catalyzed by HMG-CoA synthase. Then, HMG-CoA is cleaved to acetoacetate and acetyl-CoA by HMG-CoA lyase. Acetoacetate is reduced by D-beta-hydroxybutyrate DHase and forms D-beta-hydroxybutyrate.
-
How Diabetes associated with ketone bodies?
As mentioned above, carbodyhrate insufficiency leads to production of ketone bodies. In diabetes, insulin is unabailable and cells can not get glucose efficiently. Such condition leads to low malonyl-CoA (which is first compound in the fatty acid formation and synthesized by combination of acetyl-CoA and bicarbonate). Therefore, carnitine acyltransferase I is not inhibited and start catalyzing fatty acid degradation, producing more acetyl-CoA and further accerate the formation of ketone bodies. -
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic state (HHS, also known as nonketotic hyperglycemia) are two of the most serious acute complications of diabetes. They are part of the spectrum of hyperglycemia and each represents an extreme in the spectrum. DKA is characterized by the triad of hyperglycemia, anion gap metabolic acidosis, and ketonemia. Metabolic acidosis is often the major finding. The serum glucose concentration is usually greater than 500 mg/dL (27.8 mmol/L) and less than 800 mg/dL (44.4 mmol/L). Three ketone bodies are produced in DKA: two ketoacids (beta-hydroxybutyric acid and acetoacetic acid), and one neutral ketone (acetone). The treatment of DKA and HHS is similar, including the administration of insulin and correction of the fluid and electrolyte abnormalities that may be present, including hyperglycemia and hyperosmolality, hypovolemia, metabolic acidosis (in DKA), and potassium depletion.
Literature review
- Engineered enteroendocrine cells secrete insulin in response to glucose and reverse hyperglycemia in diabetic mice
- Genetically engineered insulin-producing K cells may act as surrogate β-cells to treat diabetes
- Several cell sources have been investigated for the development of surrogate β-cells, such as hepatocytes and neuroendocrine cells, but no satisfactory results have been obtained because these cells lack critical β-cell characteristics.
(hepatocytes express GLUT2 and glucokinase as glucose-sensing systems, but do not have secretory vesicles to control insulin release ; neuroendocrine cells possess secretory vesicles but do not have glucose-sensing systems) - They showed intestinal K cells (an enteroendocrine cell line) expressing insulin under the control of a GIP(glucose-dependent insulinotropic peptide) promoter secretes insulin in response to glucose.
- They transplantated these cells into diabetic mice and prove efficient remission of diabetes.
- Cells engineered for insulin expression should contain
- Proteases for insuline or proinsulin or express two chains (A, B) separately
- glucose-sensing system
- secretory granules that can release insulin promptly by exocytosis in response to extracellular glucose levels
System Design
This system have two major signaling subsystems (glucose and ketobodies)
- Subsystem 1: glucose-regulated insulin secretion (I)
- When concentration of blood glucose is at medium or high level, the expression of insulin will be activated
- The alert of blood glucose can help to (1) accelerate the utilization of glucose and lower the blood sugar to avoid hyperglycemia and related chronic complications, and (2) prevent the cell to break down fatty acid for energy and produce toxic ketobodies which cause DKA
- Subsystem 2: ketone body cleaning
- When acetoacetate (one of the three compound produced when fatty acid are broken down for energy instead of glucose) is detected or when concentration of blood glucose is at high level, the ketone degradation pathway will be activated
- The high level of blood glucose indicates the cell of patient may have consumed fatty acid for a period of time. Thus, the ketone cleaning pathway is NOT ONLY enhanced by acetoacetate BUT ALSO high blood glucose concentration.
- Subsystem 3: glucose-regulated insulin secretion (II)
- When glucose at low concentration, the insulin secretion would be inhitbited
- blocked by DNA-binding protein prevent promoters of insulin to be expressed
- blocked by inhibitory anti-sense RNA of insulin mRNA molecule
- blocked by insulin degradation enzyme or insulin binding protein
- When glucose at low concentration, the insulin secretion would be inhitbited
- Subsystem 4: stop glucose utilization in E.coli
- destrurction of glucose transporters to avoid the glucose intake of E. coli
- Subsystem 5: insulin (110 a.a.) delivery
- By protein sorting (signal peptide)
- By vesicle
In silico computational analysis
In the first stage of this project, we use E. coli as model to prove our concepts in the design.
- Sensing promoter finding
- To make two subsystems above possible, the first task is to find the proper sensing system for our defined signals. It is very fortune that E. coli has built in these two signal sensing pathway.
- glucose sensing
- acetoacetate sensing
- promoter found in RegulonDB (gene name: AtoD, gene_id:b2221)
- caattagttaaataactaaatccaataatctcattctggcactccccttgctattgcctgActgtacccacaacggtgtat
- Insulin expression and ketone degradation
- insulin protein (110 a.a.)
- ketone degradation in human (KEGG pathway id: 00072)
- Controlling circuit
- Insulin expression: put insulin after the glucose-sensing promoter
- ketone degradation
- duel promoter
- lac operon method
In vivo construction
- Creation of standard system in E. coli (prove of concept)
- Prove the system can work in single cell level
- Prove the system can be tuned with different sensitivity and activity as desired
- Transfer standard system to mammalian cell (close to practical use)
- trail-and-error: comparing the difference between two different host system (E. coli and mammalian cell)
- tune the system to work properly
Time Table of Lab works
- Vector preparation
- Day 1 Insertion of desired promotor into vector (enzyme digestion, ligation)
- Day 2 Transformation
- Day 4 Plasmid extraction, check with restriction mapping
- Day 5 Insertion of insulin gene (or marker) into promotor containing vector (enzyme digestion, ligation)
- Day 6 Transformation
- Day 7 Transfer the desired colony into liquid medium
- Day 8 Plasmid extraction, check with restriction mapping
- Protein level check
- Day 9 Functional assay (ELISA, Western Blot), kinetic studies
- Design on promoters
- Day 10~ Promotor optimization (site directed mutagenesis or chemical synthesis)
Optional: Check construct with DNA sequencing
P. S. Characterization of promoter activity is required before the insersion of insulin gene into the vector.
Discussion (7/13)
- How to transport insulin from intracellular side to extracellular side in E. coli?
- To prove that insulin successfully secrete to extracellular space, we engineer insulin-reporter fusion protein to visualize insulin-receptor interaction. If the attached reporter affect the function of insulin, we need a control group that express insulin only gene and measure the extracellular glucose concentration to demonstrate indirectly that the secreted insulin does interact with receptor and activate downstream signal transduction pathway.
Experiment Design
- define three promoters response for low (P50), medium (P200) and high (P400) glucose level, and promoter (Pace) respoding for acetoacetate
- GRRBS = Glucose responsive Regulator Binding Site
- construction
- P200 + signal peptide + insulin A chain + insulin B chain + Reporter 1
- P400 + signal peptide + insulin A chain + insulin B chain + Reporter 2
- Pace + beta-ketoacyl-CoA transferase (Ketone Degradation Enzyme)
- P50 + GRRBS + RNAi_INSA + RNAi_INSB
- evaulations
- insulin transport
- insulin vs. glucose
- RNAi vs. glucose
- KDE vs. ketone
- glucose vs. time
- insulin vs. time
- Ketone vs. time
References
- Engineered enteroendocrine cells secrete insulin in response to glucose and reverse hyperglycemia in diabetic mice, 2007 Mol Ther.
- use K cell which has two major characteristics similar to native insulin-secreting cell (beta cell)
- glucose sensing
- glucose transporter 2 (GLUT2)
- glucokinase (GK)
- insulin processing protease
- glucose sensing
- in vitro test
- whether the insulin secreation can response and elevate glucose level
- animal test in diabetic mice
- after transplating the engineered cell, the blood glucose level can be controlled normally within two weeks
- use K cell which has two major characteristics similar to native insulin-secreting cell (beta cell)
- Development of genetically engineered human intestinal cells for regulated insulin secretion using rAAV-mediated gene transfer, 2003
- Recombinant DNA Technology in the Synthesis of Human Insulin
- Glucose-Dependent Insulin Release from Genetically Engineered K Cells, 2000 science
- use K cells (a kind of enteroendocrine cells) in gut lining mainly located in the stomach
- the engineered K cells can release insulin in glucose-dependent manner
- they test this engineered cell in mice undergoing destruction of the native insulin-producing beta cells
- this engineered K cells can protect this mice from
- developing diabetes and
- maintain glucose tolerance
- Glucose-stimulated and self-limiting insulin production by glucose 6-phosphatase promoter driven insulin expression in hepatoma cells, 2000 Gene Therapy