From 2007.igem.org
(Difference between revisions)
|
|
| Line 74: |
Line 74: |
| | ===References=== | | ===References=== |
| | | | |
| - | ====[http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1449051&blobtype=pdf Bloor]====
| + | see [[Edinburgh/Popper/References]] |
| - | '''- An Efficient Method of Selectable Marker Gene Excision by Xer Recombination for Gene Replacement in Bacterial Chromosomes (2006)'''
| + | |
| - | | + | |
| - | : This article describes a system where a gene is deleted by replacing it with an antibiotic marker. The antibiotic marker is then excised during next cell divsion using the naturally expressed XerCD recombinases. This is similar to what we are doing except instead of excising we are inverting.
| + | |
| - | | + | |
| - | {|align="center" style="width:100%; border:2px #a3b1bf solid; background:#f5faff; text-align:left;"
| + | |
| - | |-
| + | |
| - | |"The natural dif site is in the chromosome terminus region and is essential for correct chromosome segregation at cell division: deletion of difE. coli results in a subpopulation of E. coli cells with a filamentous morphology (12). However, if natural difE. coli is deleted, another difE. coli site will not enable dimer resolution if placed outside of the 30-kb dif activity zone (DAZ) in the terminus region (6, 16). Therefore, while our method involves insertion of one or more extra dif sites at different chromosomal loci, this should not have an adverse effect on chromosome segregation, although the possibility that introduction of multiple dif sites in close proximity might result in deletion of intervening chromosomal DNA should be considered."
| + | |
| - | |}
| + | |
| - | | + | |
| - | :: '''Summary:''' Dif sites outside of the DAZ zone shouldn't cause problems with the natural dif sites of the chromosome/plasmid
| + | |
| - | | + | |
| - | ====[http://www.blackwell-synergy.com/doi/full/10.1046/j.1365-2958.1998.00651.x Kuempel]====
| + | |
| - | '''- Cell division is required for resolution of dimer chromosomes at the dif locus of Escherichia coli (1998)'''
| + | |
| - | | + | |
| - | : Good overview of the natural Xer recombinase system.
| + | |
| - | :::[[Image:Edinbugh_natural_Xer_recombinase.gif]]
| + | |
| - | :: '''Summary:''' Dimers arise when a growing plasmid recombines with its self or if a daughter chromosome breaks at a replication fork
| + | |
| - | | + | |
| - | {|align="center" style="width:100%; border:2px #a3b1bf solid; background:#f5faff; text-align:left;"
| + | |
| - | |-
| + | |
| - | |"In strains deleted for dif, failure to resolve dimer chromosomes leads to the Dif phenotype, which includes filamentation in a fraction of the cells, abnormal nucleoid morphology, SOS induction and decreased growth rate and plating efficiency compared with wild-type cells (Kuempel et al., 1991; Cornet et al., 1996)."
| + | |
| - | |}
| + | |
| - | | + | |
| - | :: '''Summary:''' Missing or broken dif sites result in filamentation and slower growth rate
| + | |
| - | | + | |
| - | {|align="center" style="width:100%; border:2px #a3b1bf solid; background:#f5faff; text-align:left;"
| + | |
| - | |-
| + | |
| - | |"dif is composed of a 28 bp ‘core’ sequence homologous to cer, which acts as the binding site for the resolvase enzymes XerC and XerD (Blakely et al., 1993). A difference between the sites is that cer is flanked by accessory sequences, which bind additional proteins and enhance recombination between sites in dimers. This provides directionality so that resolution of dimers to monomers is highly favoured (Blakely and Sherratt, 1996). Flanking sequences are not involved in resolution at dif as the phenotype of a 173 kb deletion can be suppressed by insertion of a 33 bp dif sequence (Tecklenburg et al., 1995). It has been proposed that directionality is provided by some aspect of chromosome partitioning itself. Structure is also important, as ectopic dif sites are only fully functional when located in an ≈30 kb region that surrounds the normal location of dif (Cornet et al., 1996; Kuempel et al., 1996)."
| + | |
| - | |}
| + | |
| - | | + | |
| - | :: '''Summary:''' cer sites use accessory proteins to enhance dimer->monomer versus the reverse. dif sites doen't use accessory proteins? (what about FtsK?) and favor the dimer-> monomer direction by some other structural or mechanical mechanism inherent in cell division.
| + | |
| - | | + | |
| - | ====[http://www.blackwell-synergy.com/doi/pdf/10.1111/j.1365-2958.2007.05755.x Bigot]====
| + | |
| - | '''- a literal chromosome segregation machine (2007)'''
| + | |
| - | | + | |
| - | :An up to date review on the mechanics of FtsK
| + | |
| - | | + | |
| - | :::[[Image:Edinbugh_mechanics_of_FtsK.gif]]
| + | |
| - | | + | |
| - | :: '''Summary:''' The yellow rings are the FtsK motors which powerfully pull the DNA through them. When they encounter dif sites with XerC and XerD recombinases on them the recombination event is activated
| + | |
Revision as of 10:47, 26 July 2007
https://static.igem.org/mediawiki/2007/f/f5/800px-Edinburgh_City_15_mod.JPG
Division PoPper
The idea is to use the XerC and D recombinases to invert a section of DNA at cell division. Inversion of the DNA causes a PoPS pulse as output.
Project Goal
The aim is to produce output as a function of bacterial cell division. Different versions of the recombination enable different downstream outputs. The system may for example perform programmed cell death after a set number of cell divisions. We hope to further analyse cell division and recombinase mechanisms since bacterial cell division is still relatively poorly understood.
Current Device Idea
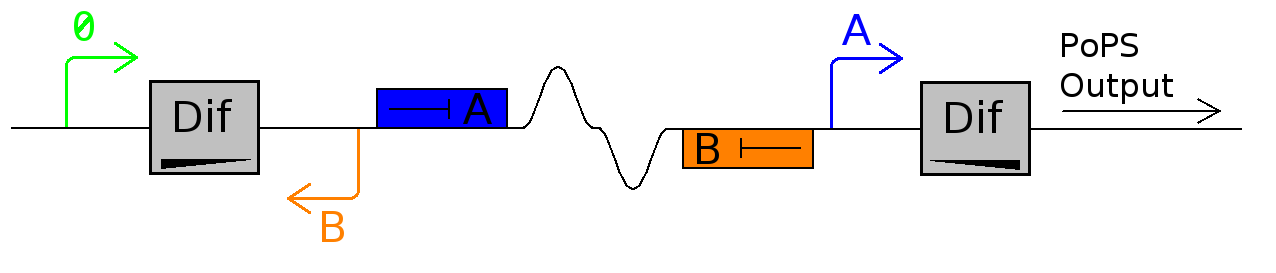
This device outputs a PoPS pulse at each cell division.
It can then be hooked up to another device such as a counter.
For this device to work, dif site-enclosed DNA needs to flip but once per division. Like all recombination sites dif-sites are directional and bacteria uses directly repeated dif-sites to resolve genome dimers. Research shows that this resolution occurs only at septation, and we use this temporal control to inverse a plasmid-kept sequence to induce differential functions.
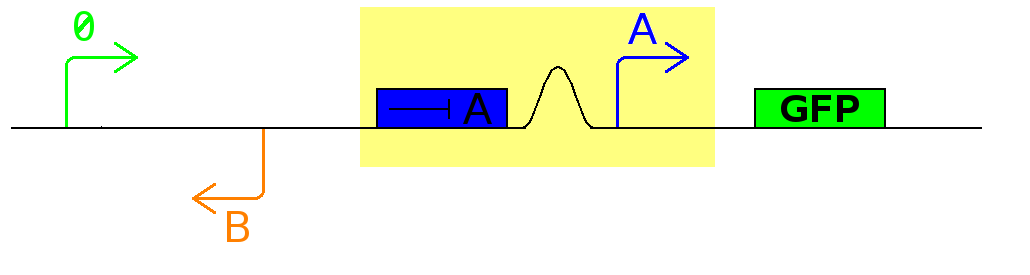
In it's current configuration, Promoter A is repressed by continually produced represser A. At this time, no represser B is produced.
As cell division occurs, DNA enclosed by two inverted dif sites is reversed. Initially there is no represser B present in the cell so promoter B produces PoPS output. As time passes, represser B is produced and 'turns off' promoter B. Meanwhile represser A is no longer produced and degrades so that, at the next division, promoter A can produce PoPS output and the process repeats.
Assumptions
- The Dif sites flip only once during cell division
- Repressors A and B are produced and degrade faster than the cell cycle
- Promoter B does not interfere with production of repressor A
Initial Experiments
As stated before, the device relies on the dif site flipping only once per cell division. To test whether or not this actually happens, we have devised two simpler experiments.
Exp 1
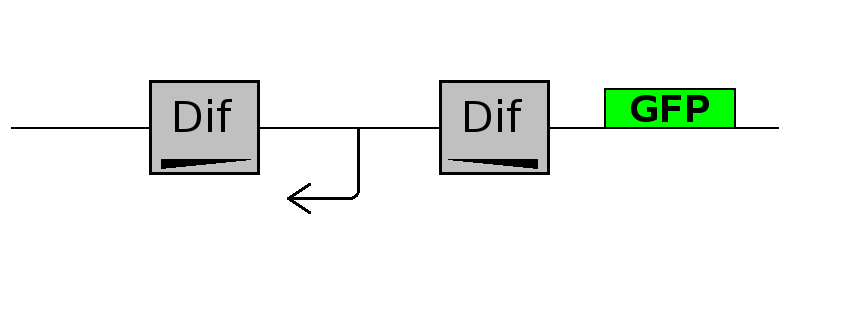
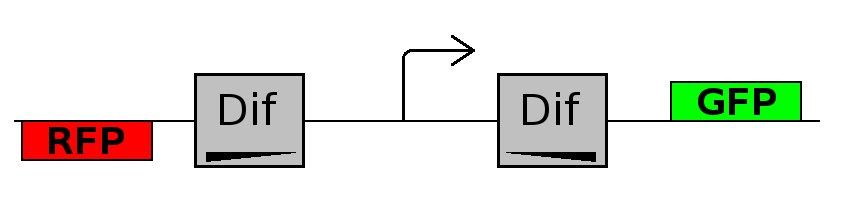
This experiment will prove whether or not the DNA between the two dif sites flip at all during cell division. If the a flip occurs, then the direction the promoter operates will be changed and GFP will be expressed.
Exp 2
This investigates the number of flips that occurs during division and will require rapidly degrading fluorescent proteins.
After each division we expect to see a change in colour as the promoter activates a different reporter.
Exp 3
Currently we are planning to test how the dif sites flip using exps 1 & 2. The final device requires a few more parts, each of which needs to be tested.
We might wish to test the repressors without the dif sites
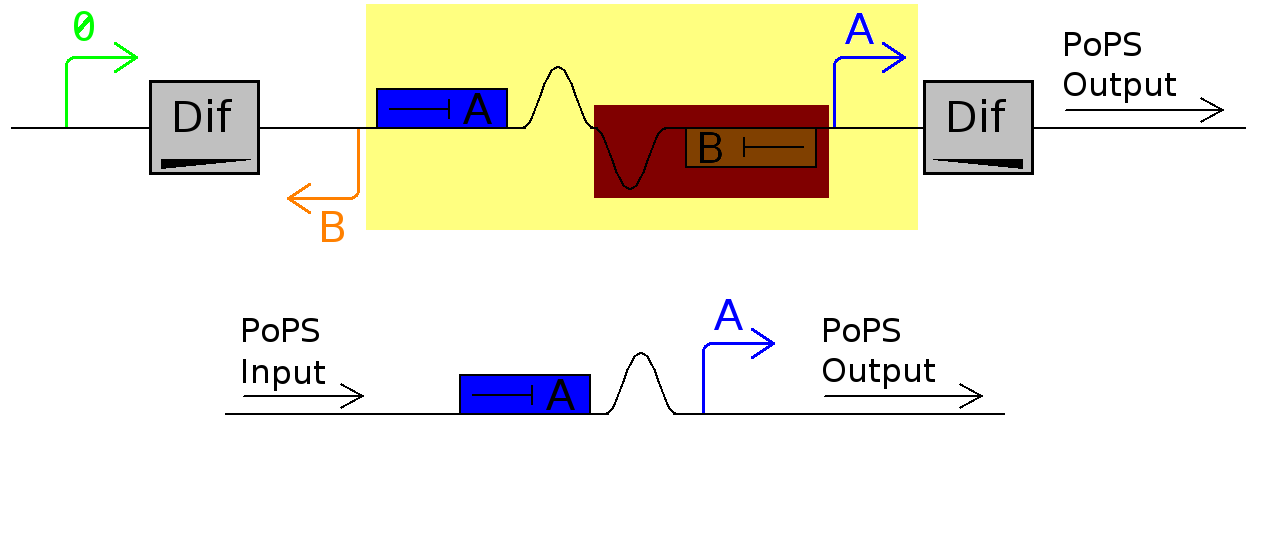
The repressor part of the system is simply a pair of inverters.
There are 3 basic types of inverter in the registry:
- TetR BBa_Q04400 “this inverter functions well. [jb, 5/24/04]”
- CI (Lambda) BBa_Q04510 “this inverter functions well. [jb, 5/24/04]”
- LacI BBa_Q04121 "a strong 'on' state with significant background in the 'off' state. [jb,5/24/04]”
Suggested exp 3
This will test to see if promoter B causes problems with promoter 0's promoting repressor A and tests the standard repressor inverter found in the registry.
Parts required:
- Promoter 0 – suggest BBa_I0500 (arabinose) – Plate 2, 9I
- Promoter B – backward lacI – Used in exp 1
- Inverter – suggested BBa_Q04510 (CI (Lambda)) – Plate 2, 13K
- Reporter – use same as experiment 1
References
see Edinburgh/Popper/References