Jamboree/DNA Submission
From 2007.igem.org
| (8 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{TOCright}} | {{TOCright}} | ||
| - | <font color=red | + | |
| + | <font color=red>'''Online DNA Submission form is now live!'''</font> <small>(--[[User:Meaganl|Meaganl]] 15:34, 18 October 2007 (EDT))</small> | ||
| + | |||
==iGEM 2007 Part Submission== | ==iGEM 2007 Part Submission== | ||
| - | In order to qualify for winning any part-related awards at the 2007 iGEM Jamboree, the teams must document their parts according to the [ | + | In order to qualify for winning any part-related awards at the 2007 iGEM Jamboree, the teams must document their parts according to the [https://2007.igem.org/Jamboree/Compete#Part_Documentation_on_the_Registry Part Documentation Guidelines] as well as submit the physical DNA to the Registry. Following these guidelines will begin the part promotion process to get your part elevated to the '''Accepted''' rating level. Only parts that are at the Accepted rating level will be considered for awards. |
| - | For iGEM 2007 we are accepting part submissions in the form of miniprepped DNA. There is an online submission form that must be completed in order to start the DNA submission process (see more details below) | + | For iGEM 2007 we are accepting part submissions in the form of miniprepped DNA. There is an [http://partsregistry.org/cgi/dna_transfer/index.cgi '''online submission form'''] that must be completed in order to start the DNA submission process (see more details below). Each part will go through a physical DNA screening/quality control process, in addition to the part documentation review process, before it is accepted into the Registry. We will also provide online status updates for each part during the submission process so that the part creator can find out at what stage of the screening process their part has gone through. |
| Line 33: | Line 35: | ||
====Part Specification page==== | ====Part Specification page==== | ||
| - | This page is the first page you will come to when you access the online submission form. You will need to specify which one of the four formats you are sending your parts in to the Registry: | + | This page is the first page you will come to when you access the online submission form. You will need to provide your user information so that we know from whom the shipment is coming from (individual and team). You will also need to specify which one of the four formats you are sending your parts in to the Registry: |
| - | + | [[Image:PCR_tube-grey2.jpg|thumb|100px|left|Single PCR tube]] | |
| - | + | '''Single PCR tube''' - If you are sending less than 4 samples, you may use the single PCR tube format to send in the physical DNA for your parts. The tube(s) must be wrapped with lab tape with the sequential number of the part written in permanent marker on tab of tape (this number is NOT the part number - it is the number of the sample i.e. #1, #2, #3, #4). When submitting 8-tube strips you must ship them in 50ml Falcon tubes. This format requires a total of 20ng of miniprepped DNA in a final volume of 10ul per sample. | |
| - | + | <br> | |
| - | + | <br> | |
| - | + | <br> | |
| - | + | [[Image:Tube strip-grey.jpg|thumb|right|8-tube strip]] '''8-tube strip''' - To send more than 4 parts at a time, use the 8-tube strip format. Wrap lab tape around the first tube in the sequence then write the part sequential number (see above) on the lab tape tab. When submitting 8-tube strips you must ship them in 50ml Falcon tubes. Each Falcon tube can hold 2 8-tube strips. ''Using the 8-tube strip format requires a total of 20ng of miniprepped DNA in a final volume of 10ul per sample.'' | |
| - | + | <br style="clear:both;"/> | |
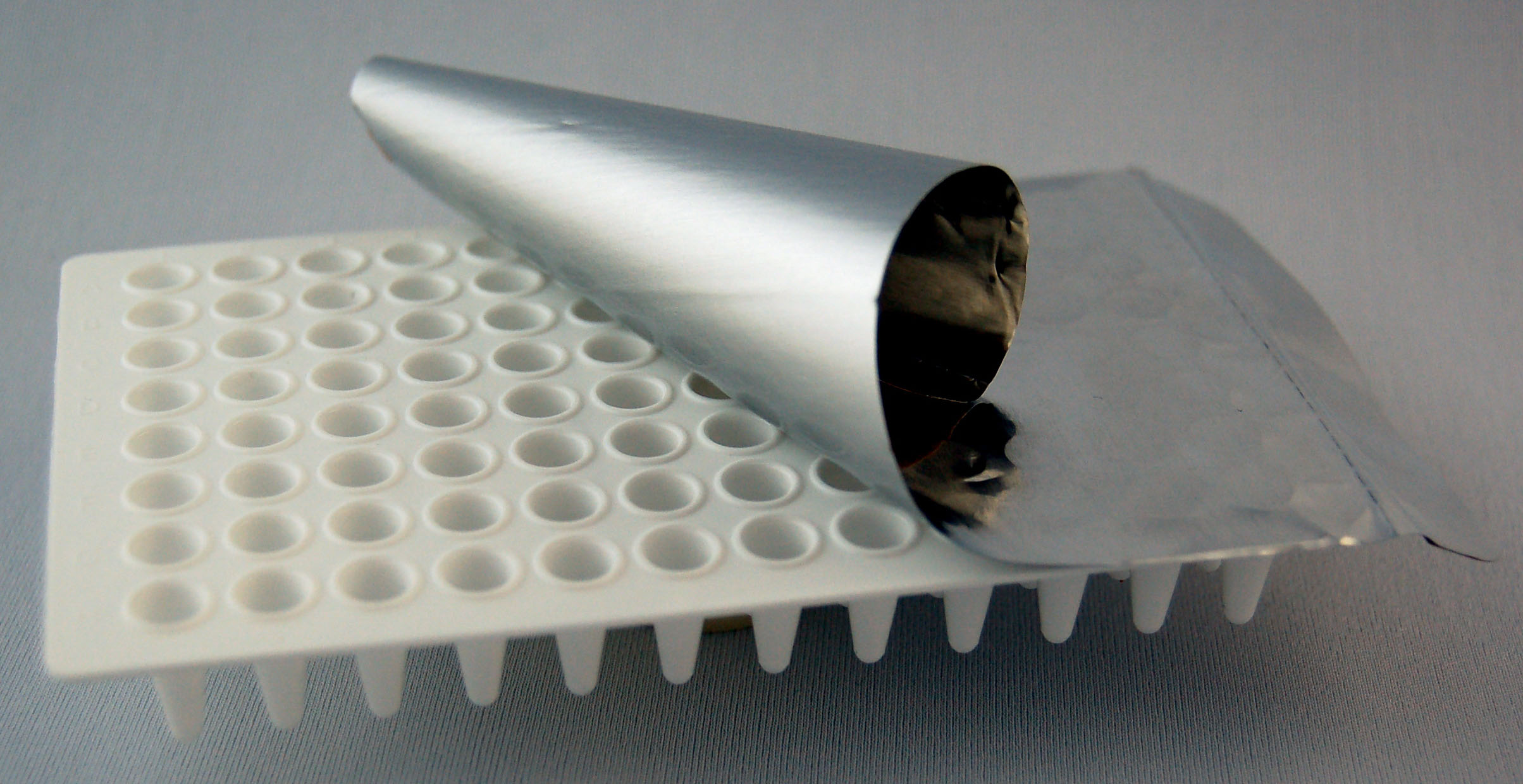
| - | + | '''96-well PCR plate''' - [[Image:96_well_plate.jpg|thumb|right|96-well plate with adhesive foil lid]]If you are submitting a large number of samples you may wish to submit the DNA in 96-well PCR plate formate. You need to add the samples in the correct order. Start with well 1A and work your way down the first column. Then continue on to well 2A and down that column. Proceed in this fashion until you have added all of the samples that you are submitting. Wrap the well 1A with lab tape to provide yourself as well as the folks at the Registry with a visible marker as to where to start processing samples. ''Using this format requires that you submit 20ng of miniprepped DNA in a final volume of 10ul per sample.'' | |
| + | <br> | ||
| + | <br> | ||
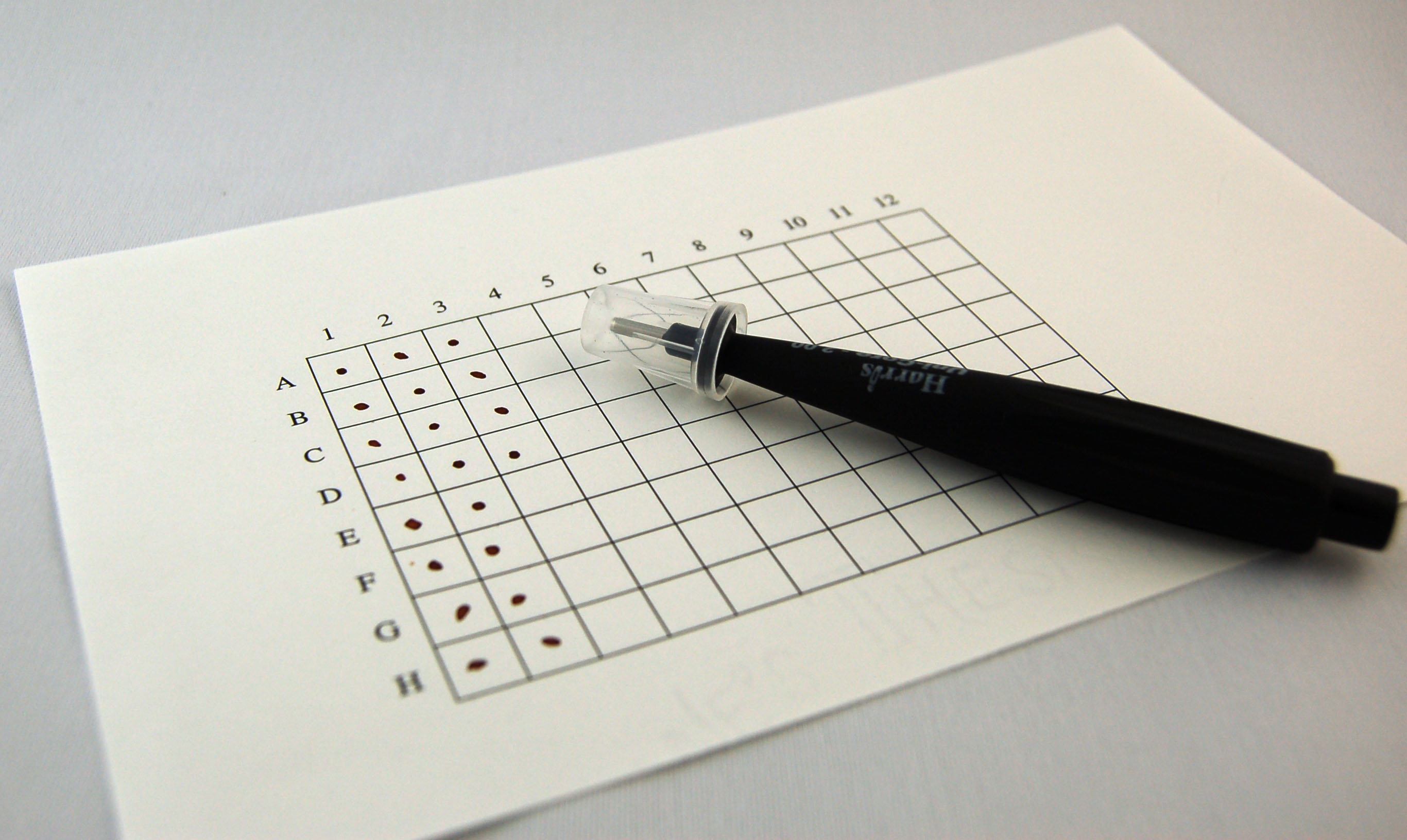
| + | '''[http://partsregistry.org/DNA_Submission_Formats/Filter_paper_grid Filter paper grid]''' - [[Image:Filter_grid.jpg|thumb|left|Filter paper grid with punching tool]]We have put a lot of effort into testing the use of high quality paper as a method by which to send DNA. The Registry will be sending each team several paper grids onto which they can spot their DNA. You must combine 1.6ul of DNA at 100ng/ul with 0.4ul of 1% Cresol Red dye and spot the resulting 2ul in the middle of the box into which you are spotting your DNA. Begin with box 1A, continue down column 1, continue on to box 2A and down that column. Proceed in this order until you have finished spotting all of the samples that you are submittin. ''You will end up submitting at least 160ng total for each sample.'' | ||
<br style="clear:both;"/> | <br style="clear:both;"/> | ||
| - | After choosing which method for DNA submission you will use, you will | + | After choosing which method for DNA submission you will use, you will continue on to specify details for each part. |
| - | |||
====Part Detail page==== | ====Part Detail page==== | ||
| - | The part detail page is where you will do just that - add all of the details for each of the parts that you will be submitting! | + | The part detail page is where you will do just that - add all of the details for each of the parts that you will be submitting! These are the criteria that we require for each part being submitted. |
*'''Part number''' - As you have already entered the part number on the preliminary part specification page, this field will be automatically filled in for you. Do double-check, however, to make sure that the part numbers that have been filled in correspond to the part numbers for which you are entering the details. | *'''Part number''' - As you have already entered the part number on the preliminary part specification page, this field will be automatically filled in for you. Do double-check, however, to make sure that the part numbers that have been filled in correspond to the part numbers for which you are entering the details. | ||
| Line 56: | Line 60: | ||
*'''Plasmid''' - You will be able to choose the plasmid that your part is in from a pull down menu that lists all of the plasmids documented on the Registry. IMPORTANT: If for some reason the plasmid that your part is in does not appear on the pull down menu you must first enter the plasmid into the Registry. Instructions on how to add a plasmid to the Registry can be found HERE. | *'''Plasmid''' - You will be able to choose the plasmid that your part is in from a pull down menu that lists all of the plasmids documented on the Registry. IMPORTANT: If for some reason the plasmid that your part is in does not appear on the pull down menu you must first enter the plasmid into the Registry. Instructions on how to add a plasmid to the Registry can be found HERE. | ||
| - | *''' | + | *'''Antibiotic resistance''' - Please provide the antibiotic resistance for each part that you provide. The software will automatically fill the entry field with what it thinks is the correct antibiotic but please double-check to make sure that it is correct. |
| - | + | ||
| - | + | ||
*'''Sequenced''' - Please tell us whether you have sequenced this DNA or not. Even you if you haven't sequenced the DNA that you are submitting, the sequence MUST be in the part documentation. See THIS PAGE for the part documentation rules. | *'''Sequenced''' - Please tell us whether you have sequenced this DNA or not. Even you if you haven't sequenced the DNA that you are submitting, the sequence MUST be in the part documentation. See THIS PAGE for the part documentation rules. | ||
| - | + | *'''Additional information:''' | |
| + | **'''Top10 toxicity''' - Top10 is our standard strain that we will be transforming each part into. Please let us know if this is going to be a problem with your parts. | ||
| + | **'''Only a plasmid''' - Tell us if you are sending just a plasmid. If so, let us know what the insert is (e.g. the insert in Registry plasmids is BBa_P1010, the ccdb "cell death" gene). | ||
| + | **'''Not a part or a plasmid''' - Please provide details in the comments section if this is the case. | ||
| + | |||
| - | ====Part Shipment Review page==== | + | ====Part Shipment Review/Shipping details page==== |
| - | After entering all of your part details you are given the opportunity to review all of the information that you are about to submit to the Registry. Take a look at all of the part details and make sure they are correct. At this point you | + | After entering all of your part details you are given the opportunity to review all of the information that you are about to submit to the Registry. Take a look at all of the part details and make sure they are correct. At this point you will provide the tracking number for the shipment of parts that you are sending the Registry. This tracking number is mandatory. It allows the ability to see whether the parts that you sent are stuck in customs, therefore delaying your part submission. Please provide the tracking number as well as the carrier that you are using, e.g. DHL 012345678. |
====Part Shipment Completion page==== | ====Part Shipment Completion page==== | ||
| Line 84: | Line 90: | ||
===Part Shipping Materials: ordering information=== | ===Part Shipping Materials: ordering information=== | ||
| - | + | Single PCR tubes: '''Axygen''' [http://www.axygen.com/jsp/coreIdDetail.jsp?coreId=MCT060 MCT-060-C-S]<br> | |
| + | 8-tube strips: '''Axygen''' [http://www.axygen.com/jsp/coreIdDetail.jsp?coreId=PCR0208 PCR-0208-C] or '''BioRad''' [http://www.bio-rad.com/B2B/BioRad/product/br_category.jsp?BV_SessionID=@@@@1422642991.1189446586@@@@&BV_EngineID=ccceaddlmhegdgkcfngcfkmdhkkdflm.0&categoryPath=%2fCatalogs%2fLife+Science+Research%2fAmplification+%7c+PCR%2fPCR+Plastic+Consumables%2fThin-Wall+PCR+Tubes+and+Caps%2fTube+and+Cap+Strips%2fLow-Profile+0.2+ml+Tube+Strips%2f&divName=Corporate&loggedIn=false&lang=English&country=HQ&catLevel=7&catOID=-33540&isPA=false&serviceLevel=Lit+Request&searchStr=TLS+0801&cateName=Ordering+Information TLS 0801]<br> | ||
| + | 96-well plates: '''VWR''' [https://obi2.vwrsp.com/catalog/product/index.cgi?catalog_number=82006-650&inE=1&highlight=82006-650&from_search=1 82006-650]<br> | ||
| + | Filter paper grid: provided by the Registry | ||
Latest revision as of 19:34, 18 October 2007
|
Online DNA Submission form is now live! (--Meaganl 15:34, 18 October 2007 (EDT))
iGEM 2007 Part Submission
In order to qualify for winning any part-related awards at the 2007 iGEM Jamboree, the teams must document their parts according to the Part Documentation Guidelines as well as submit the physical DNA to the Registry. Following these guidelines will begin the part promotion process to get your part elevated to the Accepted rating level. Only parts that are at the Accepted rating level will be considered for awards.
For iGEM 2007 we are accepting part submissions in the form of miniprepped DNA. There is an [http://partsregistry.org/cgi/dna_transfer/index.cgi online submission form] that must be completed in order to start the DNA submission process (see more details below). Each part will go through a physical DNA screening/quality control process, in addition to the part documentation review process, before it is accepted into the Registry. We will also provide online status updates for each part during the submission process so that the part creator can find out at what stage of the screening process their part has gone through.
Part Documentation Guidelines
All the documentation for your parts is expected to follow the guidelines set by the Judging Committee. The Registry will also provide more elaborate part documentation guidelines that you may want to keep in mind when documenting your parts. Following these expanded guidelines will be necessary in order for parts to start the Part Promotion process and be promoted to Accepted parts. For more information on the Registry Part Promotion process see [http://partsregistry.org/Part_Promotion_Process this Registry page] for a brief explanation. A more detailed explanation of the Part Promotion process will be provided soon.
Quality Control Process
In order to assess the quality of the parts accepted into the Registry, we will perform a standard set of experiments with a focus on verifying the length of the insert. In addition we will aim to validate the declared antibiotic resistance of each part.
The quality control process is comprised of the following tasks:
- Transform: we will transform each sample of DNA into our standard cell strain, Top10
- Pick/Inoculate single colony: after transforming the DNA into Top10 cells, we will pick a single colony and inoculate into the antibiotic specified by the creator. We will also inoculate that colony into the rest of our standard antibiotic set (Ampicillin, Chloramphenicol, Tetracycline, Kanamycin) and watch for growth in the correct antibiotic
- Miniprep : the bacterial culture grown in the specified antibiotic will be miniprepped to isolate plasmid DNA
- Digest : the miniprepped plasmid DNA will be cut with EcoRI and PstI to cut out the insert from the plasmid
- Gel: the digested plasmid DNA will be run on an Invitrogen E-gel to visualize the insert and the plasmid. From this gel we can tell whether the part length corresponds to the length designated by the designer.
If the part length as detected on the E-gel corroborates what the designer has specified in the part documentation AND if the antibiotic testing is consistent with the expected antibiotic growth pattern, the physical DNA for the submitted part will be deemed Accepted.
Online Submission Form
In order to accept physical DNA submission from teams in an organized fashion, we will be requiring teams to complete an online DNA submission form. The form is comprised of four parts:
Part Specification page
This page is the first page you will come to when you access the online submission form. You will need to provide your user information so that we know from whom the shipment is coming from (individual and team). You will also need to specify which one of the four formats you are sending your parts in to the Registry:
Single PCR tube - If you are sending less than 4 samples, you may use the single PCR tube format to send in the physical DNA for your parts. The tube(s) must be wrapped with lab tape with the sequential number of the part written in permanent marker on tab of tape (this number is NOT the part number - it is the number of the sample i.e. #1, #2, #3, #4). When submitting 8-tube strips you must ship them in 50ml Falcon tubes. This format requires a total of 20ng of miniprepped DNA in a final volume of 10ul per sample.
After choosing which method for DNA submission you will use, you will continue on to specify details for each part.
Part Detail page
The part detail page is where you will do just that - add all of the details for each of the parts that you will be submitting! These are the criteria that we require for each part being submitted.
- Part number - As you have already entered the part number on the preliminary part specification page, this field will be automatically filled in for you. Do double-check, however, to make sure that the part numbers that have been filled in correspond to the part numbers for which you are entering the details.
- Plasmid - You will be able to choose the plasmid that your part is in from a pull down menu that lists all of the plasmids documented on the Registry. IMPORTANT: If for some reason the plasmid that your part is in does not appear on the pull down menu you must first enter the plasmid into the Registry. Instructions on how to add a plasmid to the Registry can be found HERE.
- Antibiotic resistance - Please provide the antibiotic resistance for each part that you provide. The software will automatically fill the entry field with what it thinks is the correct antibiotic but please double-check to make sure that it is correct.
- Sequenced - Please tell us whether you have sequenced this DNA or not. Even you if you haven't sequenced the DNA that you are submitting, the sequence MUST be in the part documentation. See THIS PAGE for the part documentation rules.
- Additional information:
- Top10 toxicity - Top10 is our standard strain that we will be transforming each part into. Please let us know if this is going to be a problem with your parts.
- Only a plasmid - Tell us if you are sending just a plasmid. If so, let us know what the insert is (e.g. the insert in Registry plasmids is BBa_P1010, the ccdb "cell death" gene).
- Not a part or a plasmid - Please provide details in the comments section if this is the case.
Part Shipment Review/Shipping details page
After entering all of your part details you are given the opportunity to review all of the information that you are about to submit to the Registry. Take a look at all of the part details and make sure they are correct. At this point you will provide the tracking number for the shipment of parts that you are sending the Registry. This tracking number is mandatory. It allows the ability to see whether the parts that you sent are stuck in customs, therefore delaying your part submission. Please provide the tracking number as well as the carrier that you are using, e.g. DHL 012345678.
Part Shipment Completion page
Once you click on the Submit Part Shipment button to finalize your submission you will come to a page that shows that you have successfully submitted your part shipment. You will also see a review of your part details that you entered on the part detail page. At this point you may want to print this page to have a record of the parts that you have submitted. Keep track of your part shipment number and check back to see what stage your submission is in (see below for details).
Physical DNA Submission: Submission status
We want you to be fully aware of the status of your part while it is going through the Registry QC process. For this reason we are creating the ability for you to check back and see what stage your is at. You will be able to see:
- when we have received the DNA
- when the part has begun the QC process
- when the part has successfully completed the QC process
- if the part did not complete the QC process, you will see when we are waiting for you to send us another sample of the part
Part Shipping Materials: ordering information
Single PCR tubes: Axygen [http://www.axygen.com/jsp/coreIdDetail.jsp?coreId=MCT060 MCT-060-C-S]
8-tube strips: Axygen [http://www.axygen.com/jsp/coreIdDetail.jsp?coreId=PCR0208 PCR-0208-C] or BioRad [http://www.bio-rad.com/B2B/BioRad/product/br_category.jsp?BV_SessionID=@@@@1422642991.1189446586@@@@&BV_EngineID=ccceaddlmhegdgkcfngcfkmdhkkdflm.0&categoryPath=%2fCatalogs%2fLife+Science+Research%2fAmplification+%7c+PCR%2fPCR+Plastic+Consumables%2fThin-Wall+PCR+Tubes+and+Caps%2fTube+and+Cap+Strips%2fLow-Profile+0.2+ml+Tube+Strips%2f&divName=Corporate&loggedIn=false&lang=English&country=HQ&catLevel=7&catOID=-33540&isPA=false&serviceLevel=Lit+Request&searchStr=TLS+0801&cateName=Ordering+Information TLS 0801]
96-well plates: VWR 82006-650
Filter paper grid: provided by the Registry