ETHZ/Model
From 2007.igem.org
(→Final model) |
(→Final model) |
||
| Line 72: | Line 72: | ||
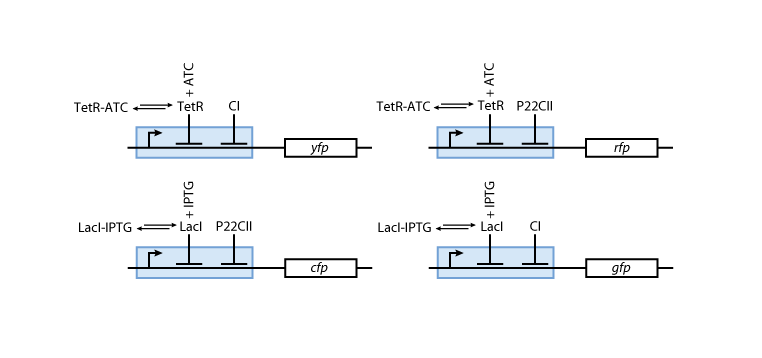
[[Image:Model03b.png|center|thumb|<b>Fig. 8</b>: The production of the florescent reporter proteins depends on the memory content (cI or p22cII) and the current input (aTc or IPTG).|600px]] | [[Image:Model03b.png|center|thumb|<b>Fig. 8</b>: The production of the florescent reporter proteins depends on the memory content (cI or p22cII) and the current input (aTc or IPTG).|600px]] | ||
| - | ==== Final model ==== | + | ==== .:: Final model ::. ==== |
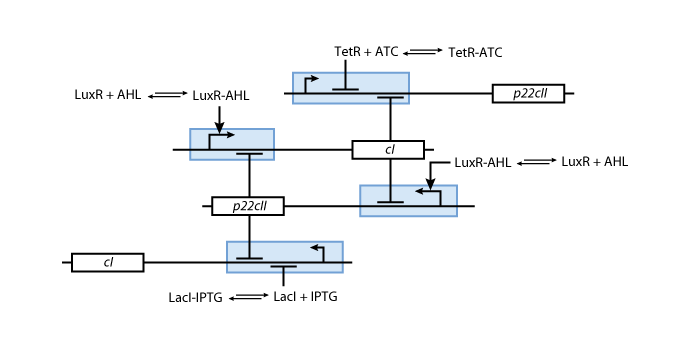
We have so far presented all the parts that are needed in order to model and simulate the behavior of a biological automaton with the ability to memorize and recognize the chemical that it is exposed to. By following the details presented in the previous section, we have all the necessary information to fully understand the interior of the black boxes that were presented in Fig. 2 and Fig. 3. Our overall system model is presented in Fig. 9. | We have so far presented all the parts that are needed in order to model and simulate the behavior of a biological automaton with the ability to memorize and recognize the chemical that it is exposed to. By following the details presented in the previous section, we have all the necessary information to fully understand the interior of the black boxes that were presented in Fig. 2 and Fig. 3. Our overall system model is presented in Fig. 9. | ||
Revision as of 21:35, 19 October 2007

.:: Modeling the educatETH E. coli System ::.
As it has been already discussed in the main page, we are interested in designing a system that is able to adapt to its environment. Our ideas were based on discussions about neural networks, and on how we can create a biological system that exhibits the behavior of learning, without having to resort to evolutionary processes.
Learning can be considered as a switching of behavior, based on some external stimuli. It comes thus naturally, to work on existing ideas of toggle switches and finite state automatons.
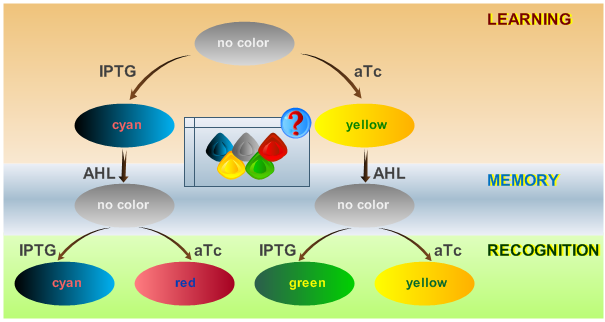
In our system, we are able to distinguish only between two chemicals. The proposed is only a minimal system that should be able to act as a proof of concept. By introducing the ability to have more final states, in an abstract manner, this correlates with the level of "intelligence" of the biological system. A protocol on how the system should react according to an input is shown in Fig. 1.
The idea behind this protocol is that
- The system will be able to learn one of two input signals - aTc or IPTG - during a learning phase if no input signal AHL is present. Depending on the input it will report by producing either green or yellow florescence.
- Once the system has learned, the inputs - aTc or IPTG - can be removed and the system goes into a memory state in the presence of the "helper" substance AHL. In this state no output color is reported. Memorizing is guaranteed by removing the input chemicals. This results in a following successful recognition phase.
- During the recognition phase, the inputs aTc or IPTG are (re-)inserted. The system reports by changing its color depending on the input and its current memory state. This is why the system has different florescent properties even in the presence of the same input. The recognition phase takes place in the presence of AHL to keep the memory enabled and avoid another learning phase. Since we would like to separate four different end states for our system, we had to use four fluorescent proteins to encode them.
.:: Model Overview ::.
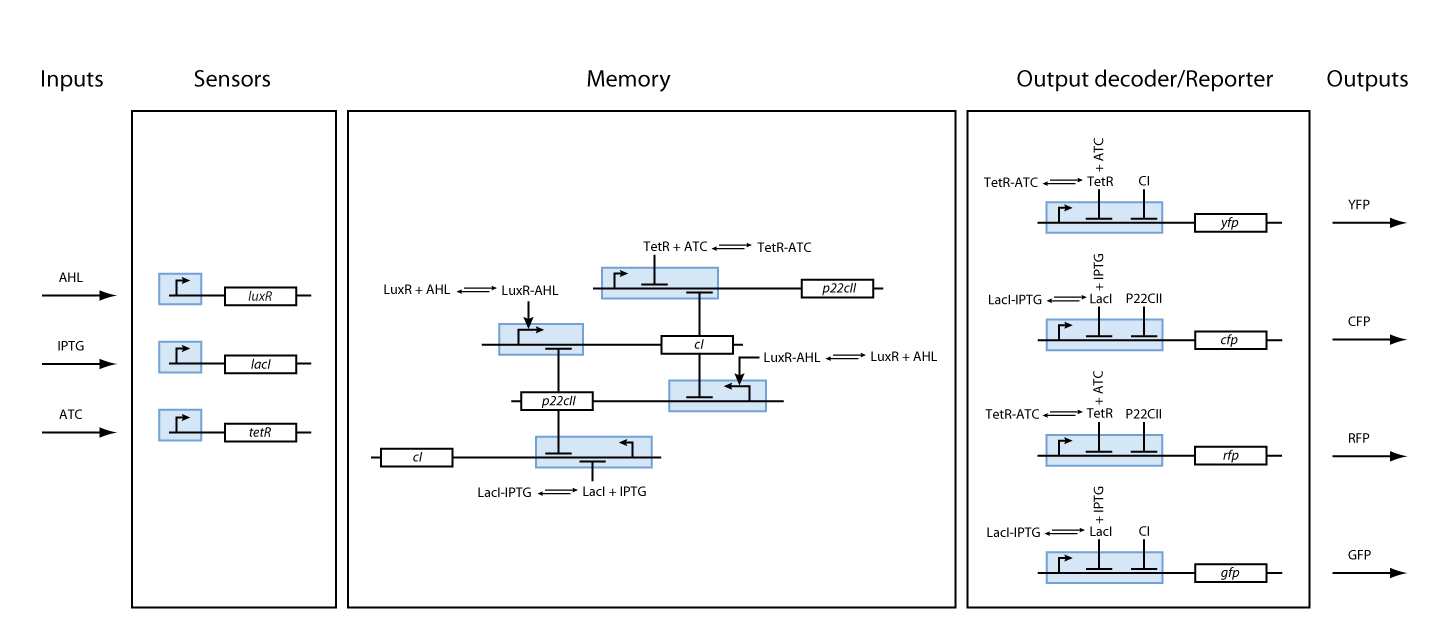
One can start developing our system with a top-down approach. We start with the classical back box approach as shown in Figure 2.
Based on what was discussed in the previous section, the properties that our system has can be summarized as follows:
- We need two inputs that should be learned/detected/adapted to,
- We need one input to switch on the memory.
- We need to alternate between at least three states. That is why we decided to use two state variables - cI and p22cII.
- We need four florescent signals for the outputs. One could also decide to take six output signals into account, to further distinguish the learning phase from the recognition phase. However, we restricted ourselves to 4 outputs to reduce the number of genes that are needed to implement the signals.
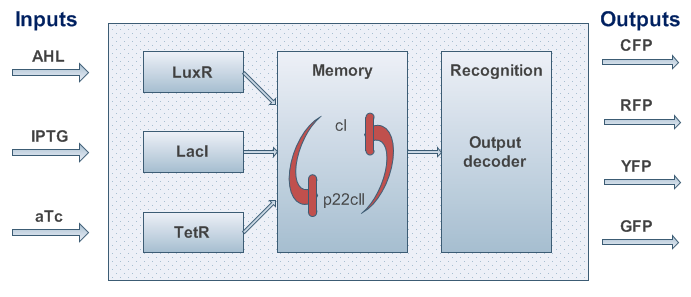
Based on the above, the internal structure of the system can be defined, and it can be seen in Figure 3. However, we had to keep in mind that the proposed system should be implemented in DNA, and that it would be sensitive to noise. As a result, we took several actions to achieve better experimental results and easier DNA construction:
- To be more robust against perturbations, we coupled the state variables cI and p22cII in the way that is well known from memory circuits. Based on this approach, one state variable is depressing the other one, and the system's internal toggle has the possibility of reaching two stable states.
- Since - due to their size - proteins can only hardly pass the cell membrane (if they are not actively transported through the cell membrane), we decided to use the much smaller inducer molecules AHL, IPTG and aTc as inputs. However, since these inducers cannot directly act on the transcription of the DNA nor on the production of proteins, we need to produce the sensor proteins LuxR, LacI and TetR that build complexes with AHL, IPTG and aTc, respectively.
- The sensor proteins and complexes are used to control the memory formation and the production of the florescent reporter proteins YFP, RFP, CFP and GFP.
.:: Detailed Model ::.
In order to test our ideas, we had to find the equations that describe the proposed model, and fill in the gaps. In this section, we are providing the details of our model, giving precise descriptions of the involved molecules and proteins. Our model is created on the basic principles of finite state machines. It is a biological automaton, that moves from the learning states, to the memorizing and recognizing states, as it was presented in Fig. 1. For a detailed analysis of the underlying finite state machine please see the section Finite State Machine View of the System.
Sensors
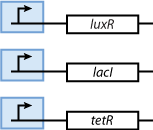
As we can observe in Fig. 3, our system is composed from three basic subparts. The first part is the part containing the sensors. Our sensors are the proteins LacI, luxR and TetR, and they are constitutively produce, in order to regulate the operation of the rest of the system. The sensing subsystem can be seen in Fig. 4.
Memory
The second subsystem is responsible for the creation and control of memories. The memory control is based on the following underlying mechanisms:
- The sensor proteins form complexes together with the inducers. These complexes are used to either activate (in case of the complex consisting of luxR and AHL) or repress (in case of the complexes consisting of LacI and IPTG as well as TetR and aTc) the DNA transcription of the proteins cI and p22cII.
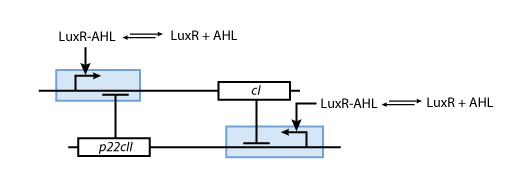
- p22cII and cI repress the DNA transcription of each other, so that the closed loop system behaves as a toggle; a dynamic system with only two possible steady states (see Fig. 6).
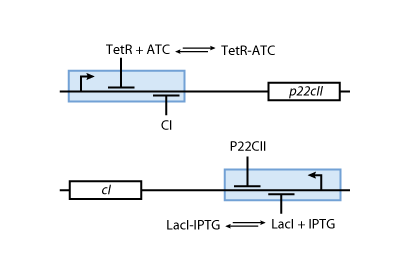
- Fig. 5 shows the protein production system that is used during the learning phase. During the learning phase, there is still no cI or p22cII produced. They are produced, only if either IPTG or aTc is added, respectively. Since no AHL is present, the inner toggle switch (see Figure 6) is turned off.
- During the memory phase, AHL is added and the IPTG and aTc are removed. That is why only the inner toggle switch (see Fig. 6) is turned on while the protein production systems shown in Fig. 5 are deactivated. Depending on what was produced during the learning phase, the production of either cI or p22cII is continued. That is why the system can act as memory, effectively storing the information that it is exposed to.
Based on all the above, we present the final assembly of the memory subsystem in Fig. 7.
Reporters
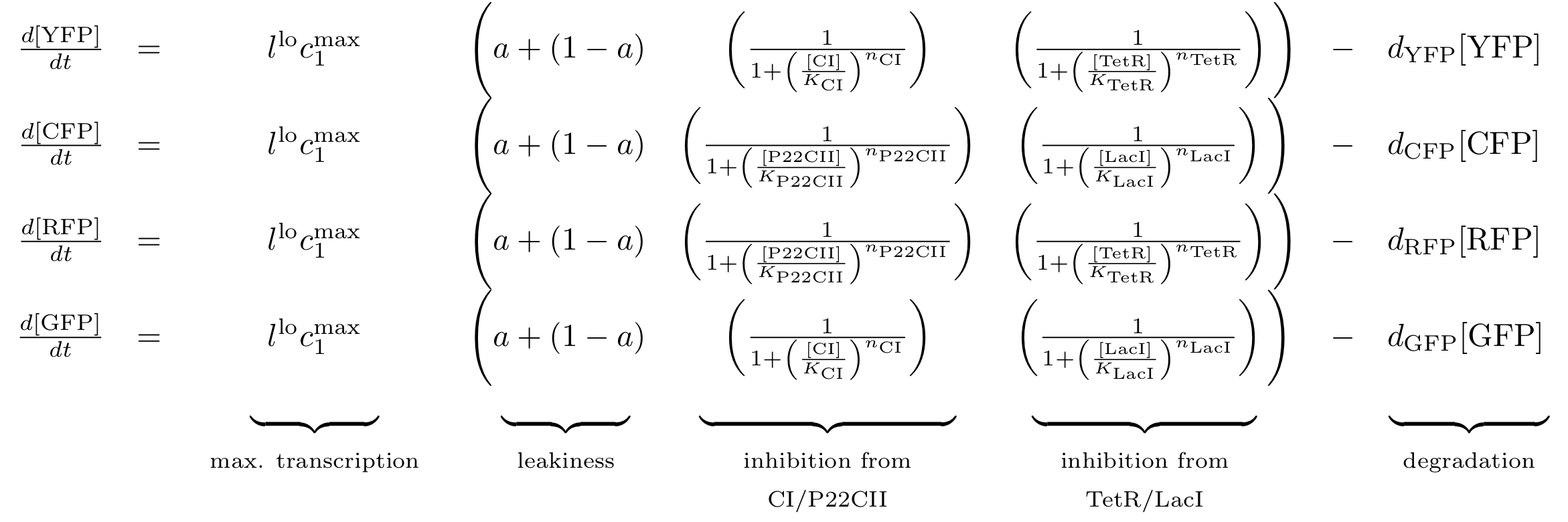
Fig. 8 gives an overview about the reporter subsystem. Florescent reporter proteins are expressed depending on the inducer concentrations, and the concentrations of cI and p22cII. For example, the presence of either TetR or cI will repress the production of YFP. However, if the inducer aTc is present, aTc will bind to TetR which can no longer block the production of YFP. We are using four fluorescent proteins, to encode the steady states of our system at the final recognition stage. This way, we are able to distinguish between all the different transition paths of our biological automaton.
.:: Final model ::.
We have so far presented all the parts that are needed in order to model and simulate the behavior of a biological automaton with the ability to memorize and recognize the chemical that it is exposed to. By following the details presented in the previous section, we have all the necessary information to fully understand the interior of the black boxes that were presented in Fig. 2 and Fig. 3. Our overall system model is presented in Fig. 9.
Equations
To perform simulations we descibe our system with the help of coupled ordinary differential equations. We use a simple notation:
- All concentrations are given in brackets like [cI].
- All decay constants are described by a variable d followed by the name of the protein they refer to.
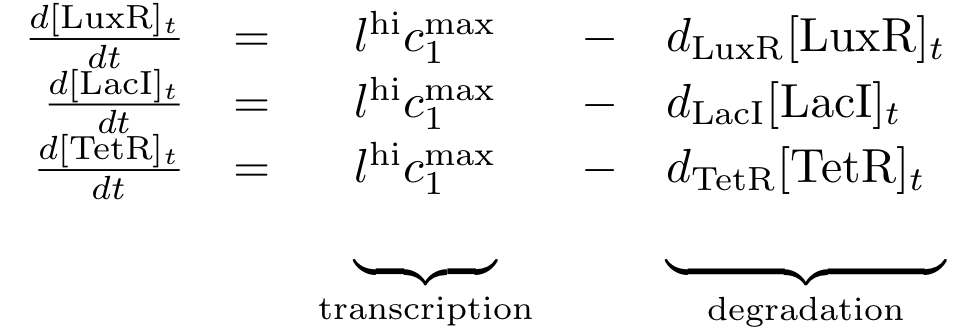
- We descibe the production of the proteins by a basic constant production level that models the leak of the production system and a factor of l and cmax that descibe the maximum production of a protein given in [M].
- Depending on if the DNA for a protein is implemented on a low or a high copy plasmid we distinguish between llo and lhi, respectively.
For a more basic introduction into how to transfer our model into equations see the section Modeling Basics.
Constitutively produced proteins
The equations for the constitutively produced proteins are very simple since we do not have to consider any dependence on other proteins. They are designed so that the protein concentration tends to lhi*cmax/d for steady state.
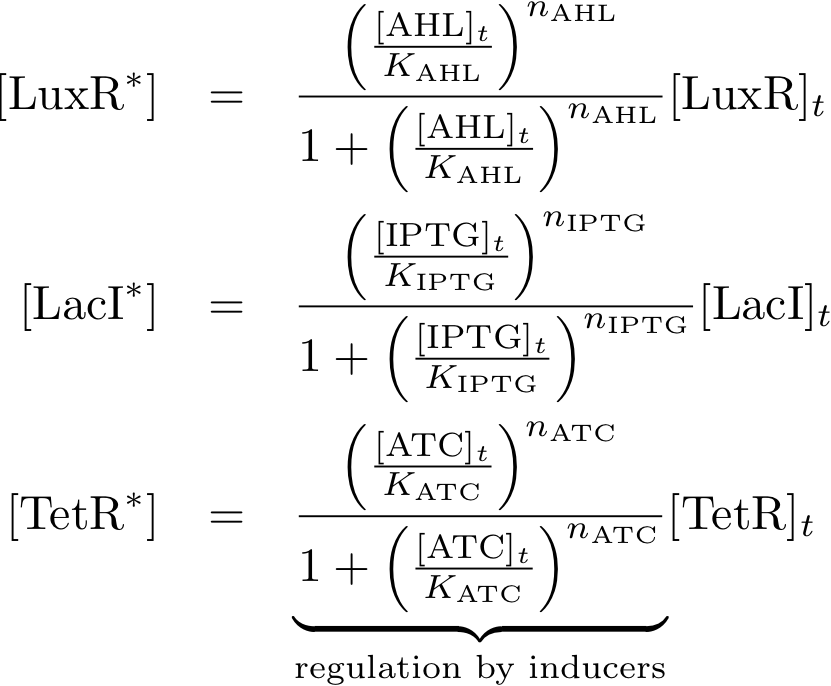
Allosteric regulation
These equations descibe the formation of complexes between the inducers and sensor proteins. We do not use differential equations but by directly descibing the concentrations of the complexes which is a valid assumption if we alway wait until steady state. We descibe the total amount of proteins by the index 't' while we use the index '*' for proteins that built a complex with the respective inducer. E.g.
- [TetRt] describes the total amount of TetR that is available while
- [TetR*] describes the proteins that are available as a complex with aTc, and finally
- [TetR] gives the concentration of free TetR proteins.
Learning and memory system
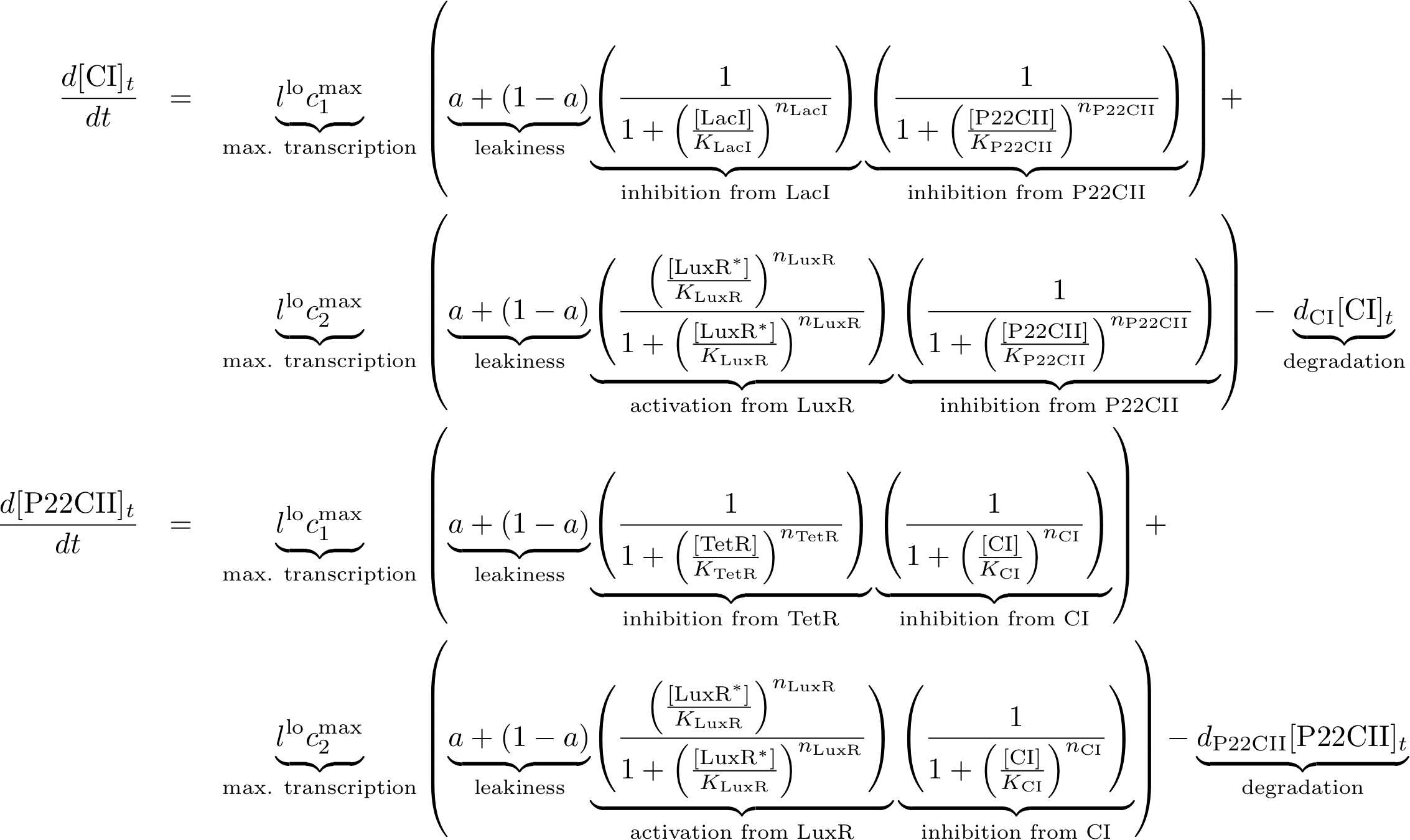
The learning and memory system is the most complicated part of our system due to the feedback between the state variables and proteins cI and p22cII as well as the variation of the production of these proteins depending on the inputs. The following equation descibe the concentrations of the memory proteins as a system of coupled differential equations. The equations consist of two major production parts and a decay part.
- The first production part models the production of either cI or p22cII during the learning phase and corresponds to the model in Figure 5.
- The second production part descibes the inner toggle switch that was shown in Figure 6.
Reporter system
The equations for the reporter system finally descibe the production of the florescence proteins depending on the inputs and memory proteins as modeled in Figure 8. Note that both inputs and memory proteins act repressively on the production of the florescence proteins. So e.g. YFP is only produced when there is both no cI and all TetR is bind in a complex together with aTc.
For information regarding how well our system performs have a look at the section Simulations.
A more detailed overview about the parameters that we used to descibe our system is given in the section Parameters.