Imperial/Dry Lab/Modelling
From 2007.igem.org
(→Formulation of the problem) |
(→Formulation of the problem) |
||
| Line 4: | Line 4: | ||
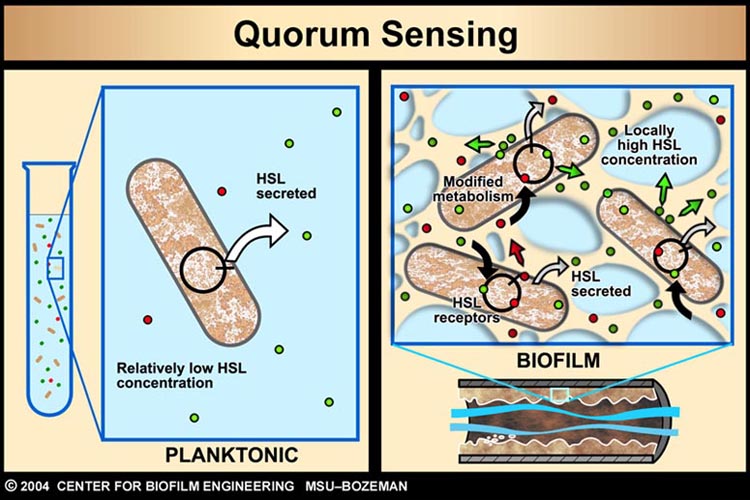
As described earlier, catheter-associated urinary tract infection (CAUTI) in the clinical setting is a prevalent problem with extensive economic impact. The underlying cause of many such infections can be attributed to the formation of biofilm, by aggregating bacteria on the surface of urinary catheters. | As described earlier, catheter-associated urinary tract infection (CAUTI) in the clinical setting is a prevalent problem with extensive economic impact. The underlying cause of many such infections can be attributed to the formation of biofilm, by aggregating bacteria on the surface of urinary catheters. | ||
| - | + | [[Image: IC07_QS.jpg|thumb| Role of AHL Quorum-sensing in biofilm formation]] | |
| - | + | ||
Infector Detector (ID) is a simple biological detector, which serves to expose bacterial biofilm. It functions by exploiting the inherent AHL production employed by certain types of quorum-sensing bacteria, in the formation of such structures.<br> | Infector Detector (ID) is a simple biological detector, which serves to expose bacterial biofilm. It functions by exploiting the inherent AHL production employed by certain types of quorum-sensing bacteria, in the formation of such structures.<br> | ||
Revision as of 21:50, 21 October 2007
Model Development for Infector Detector
Formulation of the problem
As described earlier, catheter-associated urinary tract infection (CAUTI) in the clinical setting is a prevalent problem with extensive economic impact. The underlying cause of many such infections can be attributed to the formation of biofilm, by aggregating bacteria on the surface of urinary catheters.
Infector Detector (ID) is a simple biological detector, which serves to expose bacterial biofilm. It functions by exploiting the inherent AHL production employed by certain types of quorum-sensing bacteria, in the formation of such structures.
Our project attempts to improve where previous methods of biofilm detection have proven ineffective: first and foremost, by focussing on the sensitivity of the system, to markers of biofilm: in this case, low levels of AHL production (which represents the bacterial "chatter" of such aggregating bacteria).
In doing so, a complete investigation of the level of sensitivity to AHL concentration needs to be performed - in other words, what is the minimal AHL concentration for appreciable expression of a chosen reporter protein. Furthermore, establish a functional range for possible AHL detection. How does increased AHL concentration impact on the maximal output of reporter protein?
Finally, how can the system performance be tailored, by exploiting possible state variables (e.g. varying initial LuxR concentration and/or concentration of pLux promoters).
The system performance here revolves most importantly around AHL sensitivity; however, the effect on the maximal output of fluorescent reporter protein and response time is, likewise, of great importance.