|
|
| (197 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| - | [[Image:Ncbs1.jpg|right]] | + | {|align="justify" |
| - | == '''The Company''' ==
| + | |On June 1st 2007, a group of undergraduates of various disciplines and from around the country assembled at NCBS, to attempt a 'proof of principle' demonstration. Their mission: to assemble and test a 'genetically engineered machine', a complex network assembled from simple biological parts. You will be amazed how much they managed to achieve in just six weeks! |
| | + | |[[Image:Ncbs_Logo.jpg|200px|center]] |
| | + | |- |
| | + | | |
| | + | ''The National Centre for Biological Sciences is a research institute located in Bangalore, India. In 2006, a team of graduate students from NCBS was the first from India to participate in iGEM. This year, we are very excited to compete as an all-undergraduate team of six students, from six different colleges located in four cities around India. Now that the summer is over, we hope to take the excitement of iGEM back to our home institutions, and create new teams across India who will participate in future iGEMs.'' |
| | + | |[[Image:Ncbs.jpg|400px|center]] |
| | + | |- |
| | + | | |
| | + | |align="center"|[http://www.ncbs.res.in/ National Centre for Biological Sciences, Bangalore] |
| | + | |} |
| | | | |
| - | *Mukund: Chief Mentor, Consultant and The Godfather
| + | <!--- The Mission, Experiments ---> |
| - | *Sugat: Right-hand Man and Manager of Operations
| + | |
| - | *Nilesh: The Man for all Seasons
| + | |
| - | *Vivek: Chief Troubleshooter, often his role is confused with one of a Troublemaker
| + | |
| - | *Krishna: The Sharpshooter
| + | |
| - | *Senthil: The Wikiman, also fondly referred to as the "Bio Info God"
| + | |
| - | *Varun: The Voice of Reason and Anti-Reason
| + | |
| - | *K12Z1: The Workhorse
| + | |
| | | | |
| - | == '''The Mission''' == | + | {| style="color:#294e9c;background-color:#e1e0db;" cellpadding="3" cellspacing="1" border="1" bordercolor="#1100ff" width="62%" align="center" |
| | + | !align="center"|[[Bangalore|Home]] |
| | + | !align="center"|[[The Team|The Team]] |
| | + | !align="center"|[[The Goal|The Goal]] |
| | + | !align="center"|[[Parts Data Sheets|Parts Data Sheets]] |
| | + | !align="center"|[[Mathematical Models|Mathematical Models]] |
| | + | !align="center"|[[e-Notebook|e-Notebook]] |
| | + | |} |
| | | | |
| - | To investigate multistability and hysteresis in combinatorially constructed synthetic ''Vibrio'' quorum sensing circuits
| |
| | | | |
| - | == '''The Target - ''Vibrio'' Quorum Sensing System''' ==
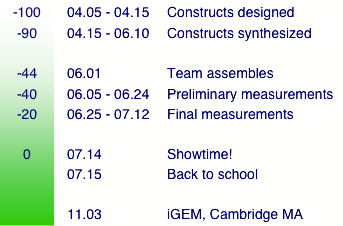
| + | [[Image:timeline.png|center|]] |
| | | | |
| - | [[Image:vibrio_qs.jpg|thumb|200px|Fig. 1: Quorum sensing in Vibrio fischeri - a LuxI-R signalling circuit. Red triangles indicate the autoinducer that is produced by LuxI. OM, outer membrane; IM, inner membrane.]]
| + | == == |
| | | | |
| - | Quorum sensing is a phenomenon by which bacteria sense a critical cell density before turning on the expression of certain genes. It involves the gradual build-up of a chemical termed the 'autoinducer' in the cell. The autoinducer freely diffuses across the cell membrane and hence, its concentration is population density dependent. When the concentration crosses a threshold, the bacteria switch to a different physiological state such as bioluminescence, virulent gene expression, and bio-film formation.
| + | <font size="2"> |
| | | | |
| - | In ''Vibrio fischeri'', when the population density (and hence the concentration of autoinducer) crosses a certain threshold, the expression of a set of genes that is required for bioluminescence is turned on. The production of the autoinducer is under the control of a gene, the expression of which involves ''positive feedback''. Figure 1 shows the various components of this system (Ref. 1).
| + | [[Image:igem_bangalore_07.jpg|thumb|550px|center|<font color="green">'''Top to Bottom''':</font><font color="black"> Navneet, Nilesh, Vivek, Vini, Krishna, Varun, E.coli, Shashanka, Mukund, Senthil and Sushant at the NCBS Amphitheatre.</font>]] |
| - | | + | </font> |
| - | ==='''Why did we pick this system?'''===
| + | |
| - | | + | |
| - | The Vibrio quorum sensing system involves a well-defined set of genes and a promoter, and has a degree of complexity that offers wide scope for exploration. Additionally, the concentration of the active transcriptional regulator, LuxR* is dependent on 3 factors:
| + | |
| - | i) The concentration of LuxI
| + | |
| - | ii) The population density
| + | |
| - | iii) The concentration of LuxR
| + | |
| - | | + | |
| - | All these factors can be experimentally controlled. Thus, the nature of the system offers one an extremely good handle on the feedback strength of the genetic circuit.
| + | |
| - | | + | |
| - | =='''A note on Multistability and Hysteresis'''==
| + | |
| - | [[Image:Break_feedback_loop.jpg|thumb|200px|left|Fig. 2: Schematic view of a feedback system before (Left) and after (Right) breaking the feedback loop. ‘ω’ is the input of the open-loop system and ‘η’ is the output.]] | + | |
| - | | + | |
| - | In the recent past, multistability has been an important recurring theme in studies on cell signalling. Angeli et al (Ref. 2) have shown that for a class of feedback systems of arbitrary order, the stability properties and bifurcation diagram of the system can be deduced mathematically from how the system behaves when feedback is blocked. [[Image:Io_curve.jpg|thumb|200px|right|Fig. 3: Steady state I/O static characteristic curve for the open loop of the mutually inhibitory Cdc2-Cyclin B/Wee1 feedback system (red). The solid blue line represents η as a function of ω for unitary feedback. ]]The system is guaranteed to be bistable for some range of feedback strengths provided the feedback-blocked system is monotone and shows a sigmoidal characteristic.
| + | |
| - | | + | |
| - | A simple graphical method can be used to deduce the stability behaviour of such systems (Ref. 2). The key feature of this approach is to view the positive feedback system as a feedback closure of its corresponding 'open loop' system. This open loop system is obtained by breaking the feedback loop at the point of feedback (Fig. 2).
| + | |
| - | | + | |
| - | Now, one can experimentally manipulate the amount of input (ω) and monitor the output (η) as a function of ω. The fixed points of the corresponding closed loop system are then obtained by intersecting η = f (ω) with the straight line, η = (1/ν) ω, where ' ν ' is the feedback strength. At these points of intersection, the open loop system exactly mimics the closed loop system. As shown in Fig. 3, they represent two stable steady states (I and III) and one unstable steady state (II).
| + | |
| - | | + | |
| - | [[Image:Bifurcation.jpg|thumb|185px|left|Fig. 4: Bifurcation diagram, showing bistability when the feedback strength ‘ν’ is between ~0.83 and ~1.8. ]]
| + | |
| - | | + | |
| - | All positive-feedback, multistable systems show the associated property of ''hysteresis''. This can be explained by analysing the corresponding bifurcation diagram, which is a plot of the steady states as a function of the feedback strength (see Fig. 4). To do this, one can vary the feedback strength, ‘ν’ and find the different points at which the I/O characteristic curve intersects the equivalence line η = (1/ν) ω. At high and low feedback strength values, the system is monostable whereas in the intermediate region, there are three intersections, one associated with an unstable state and the other two with stable states.
| + | |
| - | | + | |
| - | The bifurcation diagram clearly shows the hysteretic (i.e history-dependent) behaviour of the system in the bistable region. Increasing ν from low to high results in picking the lower branch in the bistable regime, whereas decreasing from high to low takes the system to the upper branch.
| + | |
| - | | + | |
| - | =='''Our Approach'''==
| + | |
| - | [[Image:The_Y.jpg|thumb|200px|right|Fig. 5. Diagram showing the two ways( indicated by --->) in which the loop is closed.]]
| + | |
| - | We plan to conduct experiments to explore the multistability of our synthetic genetic circuits by using the approach described by Angeli et al. We use a positive-feedback system, whose open loop can be closed in two different ways
| + | |
| - | (Fig. 5).
| + | |
| - | | + | |
| - | The transcriptional regulator, LuxR* governs the expression of the promoter, pR. Since the concentration of LuxR* depends on the concentrations of both AI and LuxR, we have a remarkable handle on the feedback. Hence, we look for bistability by varying the concentrations of AI and LuxR and shifting our I/O characteristic curves to intersect the equivalence η = ω (ν=1) line at various points. In contrast, Angeli et al varied the feedback strength (ν) values to explore bistability in their numerical simulations.
| + | |
| - | | + | |
| - | == The Parts ==
| + | |
| - | | + | |
| - | '''I''' '''''Promoters''''':
| + | |
| - | | + | |
| - | *pR
| + | |
| - | *pLac
| + | |
| - | *pTet
| + | |
| - | | + | |
| - | '''II''' '''''Regulator''''':
| + | |
| - | *LuxR
| + | |
| - | | + | |
| - | '''III''' '''''Signallin'''g'':
| + | |
| - | *LuxI --> AI
| + | |
| - |
| + | |
| - | '''IV''' '''''Inducers''''':
| + | |
| - | *IPTG (Isopropyl β-D-1-thiogalactopyranoside)
| + | |
| - | *aTc (anhydro tetracycline)
| + | |
| - | | + | |
| - | '''IV''' '''''Reporters''''':
| + | |
| - | *CFP(C)
| + | |
| - | *YFP(Y)
| + | |
| - | | + | |
| - | == '''The Main Constructs''' ==
| + | |
| - | [[Image:NET1_closed.jpg|thumb|left|200px|Fig.6 Network 1 by combinatorial construction]] [[Image:NET2_closed.jpg|thumb|right|200px|Fig.7 Network 2 by combinatorial construction]]
| + | |
| - | | + | |
| - | | + | |
| - | By combinatorial construction, we have used our network parts to design two positive feedback systems(Fig.5 & 6).
| + | |
| - | | + | |
| - | | + | |
| - | The feedback-blocked, open loop system for both the systems can be represented by using the following two constructs.
| + | |
| - | | + | |
| - | *pTet LuxI.cfp
| + | |
| - | | + | |
| - | *pLac LuxR.Y pR cfp
| + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | | + | |
| - | =='''Experiments Designed'''==
| + | |
| - | We break the closed loop of network 1 as shown in the Fig. 7.
| + | |
| - | | + | |
| - | [[Image:NET_open.jpg|thumb|200px|right|Fig.7 Diagram illustrating the feedback blocked system of Network 1]]
| + | |
| - | | + | |
| - | ==='''The open-loop system for Network 1'''===
| + | |
| - |
| + | |
| - | The feedback step involving production of LuxR from the pR promoter is broken and in its place, LuxR is placed under the regulation of the pLac promoter and the CFP gene is placed under the regulation of the pR promoter.
| + | |
| - |
| + | |
| - | Cells containing the construct pTet luxI.cfp will be initially cultured at a desired aTc concentration (to drive the production of a desired concentration of AI by inducing LuxI expression) at a particular density. The cells will then be separated by centrifugation and the medium containing AI will be added to the growth medium of the cells hosting the construct pLac luxR.yfp pR cfp
| + | |
| - | | + | |
| - | The fluorescent intensities of the reporter proteins (LuxR.YFP and CFP) will then be obtained using FACS, which will now, correspond to a single aTc concentration and cell density.
| + | |
| - | | + | |
| - | The experiments will be repeated for varying values of aTc concentrations and cell densities to obtain a family of curves relating the CFP and LuxR.YFP as shown.
| + | |