Imperial/Wet Lab/Results/ID2.1
From 2007.igem.org
m |
m (→Results) |
||
| Line 12: | Line 12: | ||
==Results== | ==Results== | ||
{|align="center" | {|align="center" | ||
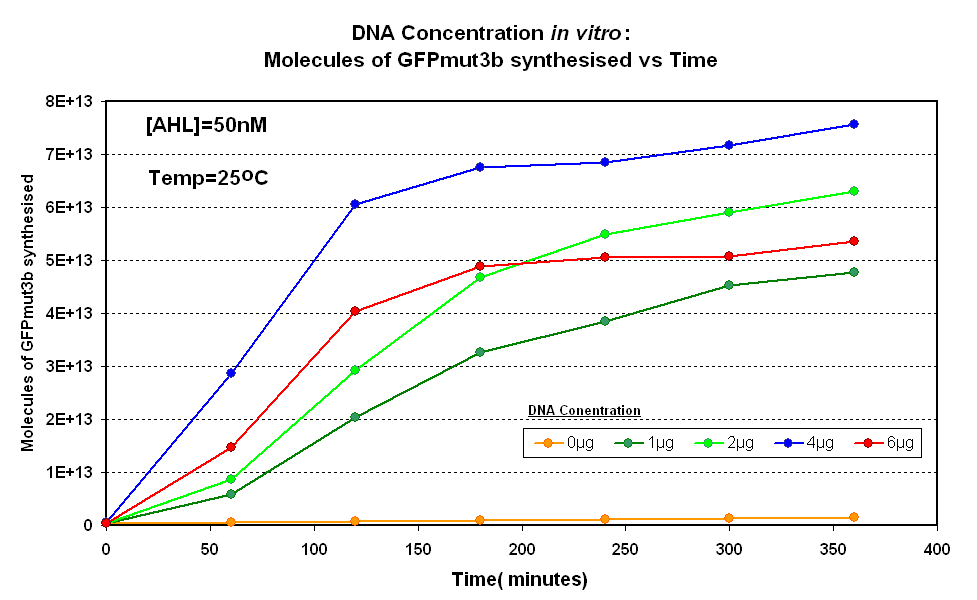
| - | | width="600px"|<br>[[Image:DNA Concentration.PNG|thumb|600px|Fig.1.1:Molecules of GFPmut3b synthesised over time, for each DNA Concentration ''in vitro'' - The fluorescence was measured over time for each experiment and converted into molecules of GFPmut3b ''in vitro'' using our calibration curve.]] | + | | width="600px"|<br>[[Image:IC 2007 DNA Concentration.PNG|thumb|600px|Fig.1.1:Molecules of GFPmut3b synthesised over time, for each DNA Concentration ''in vitro'' - The fluorescence was measured over time for each experiment and converted into molecules of GFPmut3b ''in vitro'' using our calibration curve.]] |
|} | |} | ||
{|align="center" | {|align="center" | ||
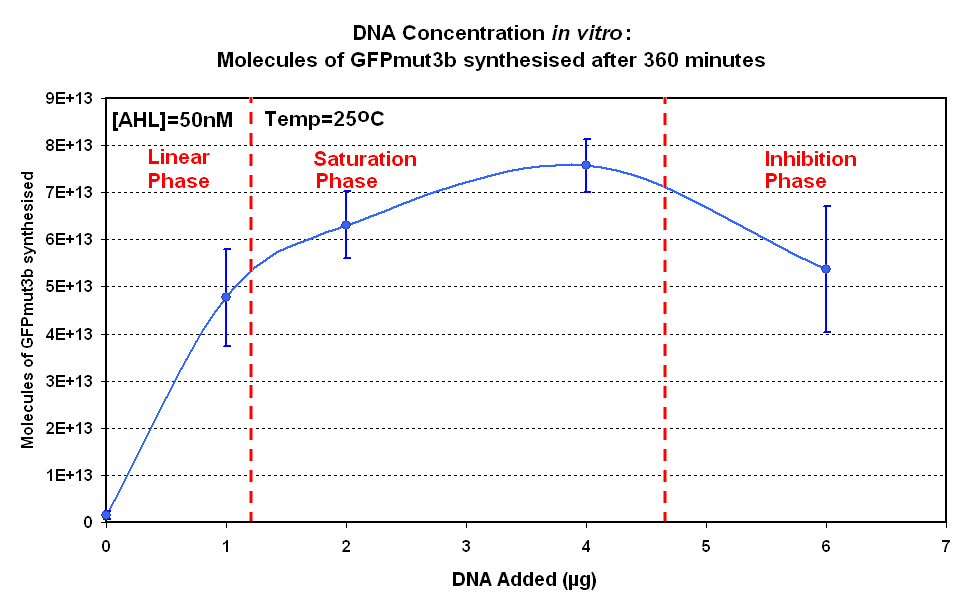
| - | | width="600px"|<br>[[image:DNA Concentration 360mins.PNG|thumb|600px|Fig.1.2:Molecules of GFPmut3b synthesised for each DNA Concentration ''in vitro'', after 360 minutes.]] | + | | width="600px"|<br>[[image:IC 2007 DNA Concentration 360mins.PNG|thumb|600px|Fig.1.2:Molecules of GFPmut3b synthesised for each DNA Concentration ''in vitro'', after 360 minutes.]] |
|} | |} | ||
Revision as of 13:38, 25 October 2007
Testing DNA concentration of pTet-LuxR-pLux-GFPmut3b In vitro
Aims
To determine the optimum concentration of [http://partsregistry.org/Part:BBa_T9002 pTet-LuxR-pLux-GFPmut3b] in vitro.
Materials and Methods
Refer to protocols page.
Tested on (Insert link)
Results
Controls:
- Negative Control- Nuclease Free Water was added instead of DNA
Constants:
- Temperature - 25°C
- Total Volume - 60µl
Raw Data
Discussion
Figure 1.1 and Figure 1.2. shows us that the synthesis of GFPmut3b increases with DNA added up until 4µg of DNA. Above this the number of molecules of GFPmut3b produced decreases.
Another interesting observation is that the 6µg of DNA begins producing GFPmut3b greater than 2µg but then levels of before 2µg. This is thought to be because the data displayed on the figures are averages of 2 samples, one of the samples levels off sooner than the other and so brings the average down. However, the results do tell us that 6µg is lower than the 4µg, therefore the 4µg is the optimum DNA for our in vitro chassis.
This agrees with promegas guide on using the commcerial cell extract in vitro chassis. The guide states that 4µg is the maximum and above this there is problems with premature termination of translational products
Conclusion
To conclude the following approximations can be made:
- Optimum DNA concentrations - 4µg of DNA