Tristable
From 2007.igem.org
(Difference between revisions)
| (41 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
<!-- | <!-- | ||
| Line 15: | Line 16: | ||
<html> | <html> | ||
<style> | <style> | ||
| + | a:link{ | ||
| + | |||
| + | text-decoration:none; | ||
| + | |||
| + | color:#4b8ada | ||
| + | |||
| + | } | ||
| + | |||
| + | |||
| + | |||
| + | a:visited{ | ||
| + | |||
| + | text-decoration:none; | ||
| + | |||
| + | color:#4b8ada | ||
| + | |||
| + | } | ||
| + | |||
| + | |||
| + | |||
| + | a:active{ | ||
| + | |||
| + | text-decoration:none; | ||
| + | |||
| + | color:#4b8ada | ||
| + | |||
| + | } | ||
| + | |||
| + | |||
| + | |||
| + | a:hover{ | ||
| + | |||
| + | text-decoration:underline; | ||
| + | |||
| + | color:#4b8ada | ||
| + | |||
| + | } | ||
body { | body { | ||
margin:0px 0px 0px 20px | margin:0px 0px 0px 20px | ||
| Line 68: | Line 106: | ||
| + | '''Click on the title to learn more about each section.''' | ||
| + | |||
| + | |||
| + | |||
| + | =[[/Intro to Tristable | Tri-Stable Toggle Switch]]= | ||
| + | [[Image:Tristable_Toggle_Switch_2007.jpg|thumb|right]]A trinary memory unit. A genetic circuit. A proof of concept. Here is the man-made architecture of the switch and the natural context of our parts. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | =[[/Modeling | Tri-Stable Model]]= | ||
| + | [[Image:beta values.png|thumb|right]]While Gardner et al created a mathematical model and a genetic switch in the Bistable paper, one was not used to design and improve upon the other. Here we extend our model to the Tristable system and discuss what certain parameters mean in terms of DNA and Proteins, Production, Repression, etc. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | =[[/Testing Constructs | Testing Constructs]]= | ||
| + | [[Image:betaTest.png|thumb|right]]The dirty side of the switch. Testing constructs that will enable us to determine our constants in absolute terms and apply a mathematical basis to changes we make on the Tri-Stable Switch architecture. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | =[[/Appendix | Appendix]]= | ||
| + | More information about where we are going and where we have been. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <!-- | ||
=Tri-stable Toggle Switch= | =Tri-stable Toggle Switch= | ||
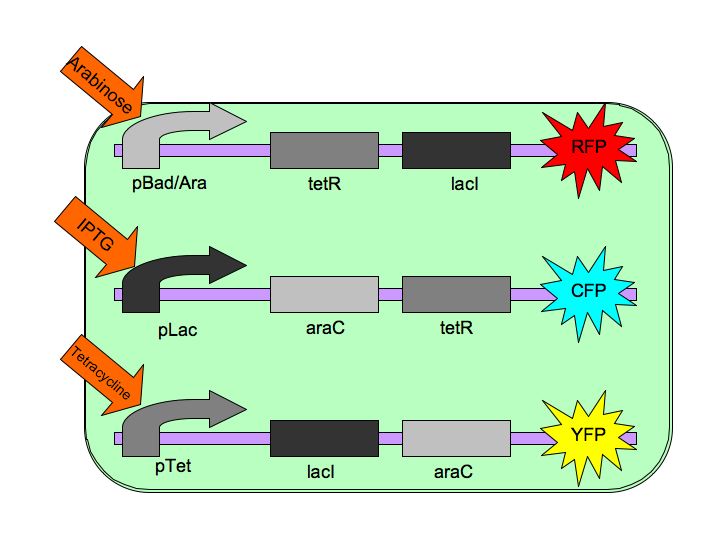
| - | The | + | The Tri-Stable switch three distinct and stable outputs in response to three distinct inputs. These three inputs are three separate chemicals which will each induce one state of the switch. [[Image:Tristable_Toggle_Switch_2007.jpg|thumb|left|The Tri-stable Toggle Switch Architecture]] In order to achieve this goal, we are constructing three constructs, each of which consists of a repressible, constitutively-on promoter attached to two repressors. Specifically, our three constructs are: |
pBAD->LacI->TetR, | pBAD->LacI->TetR, | ||
| Line 81: | Line 173: | ||
Each of the three repressors are inactivated by one of three chemicals, the three inducer chemicals mentioned earlier. These three([http://en.wikipedia.org/wiki/Arabinose arabinose], [http://en.wikipedia.org/wiki/IPTG IPTG] (Isopropyl β-D-1-thiogalactopyranoside) and [http://en.wikipedia.org/wiki/Tetracycline Tetracycline], respectively), cause conformational changes in their respective repressor proteins which keeps them from binding to DNA in an inhibitory manner which leads to gene expression. For example, in the presence of arabinose, AraC cannot repress pAraC/BAD so LacI and TetR are produced which in turn repress pTet and pLac and the pAraC/BAD construct is turned on. | Each of the three repressors are inactivated by one of three chemicals, the three inducer chemicals mentioned earlier. These three([http://en.wikipedia.org/wiki/Arabinose arabinose], [http://en.wikipedia.org/wiki/IPTG IPTG] (Isopropyl β-D-1-thiogalactopyranoside) and [http://en.wikipedia.org/wiki/Tetracycline Tetracycline], respectively), cause conformational changes in their respective repressor proteins which keeps them from binding to DNA in an inhibitory manner which leads to gene expression. For example, in the presence of arabinose, AraC cannot repress pAraC/BAD so LacI and TetR are produced which in turn repress pTet and pLac and the pAraC/BAD construct is turned on. | ||
| - | |||
| - | |||
| Line 90: | Line 180: | ||
[[Image:Two_Dimers_of_AraC.jpg|thumb|left|Dimer structure with arabinose on the left (yellow)]] | [[Image:Two_Dimers_of_AraC.jpg|thumb|left|Dimer structure with arabinose on the left (yellow)]] | ||
[[Image:AraC_Promoters.gif|left|thumb|The left image shows the araC dimer repressing transcription, while the right conformation enables transcription]]The protein forms a dimer with and without arabinose but the structural change activates or represses the pAraC/BAD. | [[Image:AraC_Promoters.gif|left|thumb|The left image shows the araC dimer repressing transcription, while the right conformation enables transcription]]The protein forms a dimer with and without arabinose but the structural change activates or represses the pAraC/BAD. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 109: | Line 188: | ||
| + | ==LacI== | ||
| + | In nature, LacI represses pLac which promotes the LacYZA genes that metabolize lactose. Thus LacI represses pLac except in the presence of lactose (or lactose mimics, eg IPTG). [[Image:LacI_repressor.gif|thumb|left|Image[http://www.mun.ca/biochem/courses/3107/Topics/Lac_genetics.html]. LacI forms a tetramer and represses pLac. However, an inducer, such as IPTG, causes a conformation change that removes LacI from the operator site.]] Lactose causes a conformational change which inhibits LacI from binding to the operator site of pLac. Four LacI proteins form a tetramer to inhibit pLac and four inducer molecules are required to cause the full conformational change in the repressor.[http://www.mun.ca/biochem/courses/3107/Topics/Lac_genetics.html] | ||
| Line 118: | Line 199: | ||
| + | ==TetR== | ||
| + | TetR represses the constitutive promoter pTet. In the presence of tetracycline, an antibiotic, a conformational change in TetR inhibits the protein from binding to the operator region. In nature, pTet promotes TetR and TetA. The latter of which acts to pump tetracycline out of the cell, thus the pump is only activated in the presence of Tetracycline.[http://en.wikipedia.org/wiki/Tetracycline_controlled_transcriptional_activation] | ||
| + | The TetR, as it turns out is a very tight repressor and a range of 0 to 1 ug/ml has been shown to cause a 5 order of magnitude change in luciferase production.[http://www.ncbi.nlm.nih.gov/sites/entrez?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=1319065&query_hl=1&itool=pubmed_docsum] | ||
| + | [[Image:Tc_bound_to_TetR.jpg|thumb|left|A tetracycline molecule binds to each of the two TetR monomers to form a dimer]] | ||
=Modeling= | =Modeling= | ||
| Line 165: | Line 250: | ||
| - | + | ===Stability=== | |
[[Image:tri stableRegion.png|left|thumb|Tri-Stable region solved in Matlab]] | [[Image:tri stableRegion.png|left|thumb|Tri-Stable region solved in Matlab]] | ||
| Line 176: | Line 261: | ||
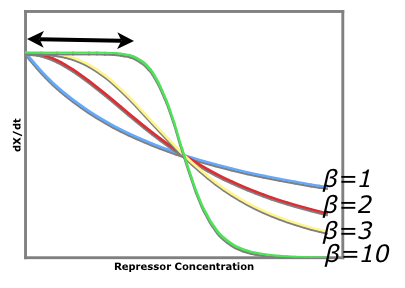
The black arrow shows that there is a larger region for larger beta values where stray repessors will not significantly affect the rate of repressor production rate whereas in a system of beta = 1, stray repressors will significantly change the system, making it less robust. | The black arrow shows that there is a larger region for larger beta values where stray repessors will not significantly affect the rate of repressor production rate whereas in a system of beta = 1, stray repressors will significantly change the system, making it less robust. | ||
| - | + | =Testing Constructs= | |
There are two methods we could follow in designing the Switch. We could randomly try different RBSs, hope it works and if not try again without having much of an understanding of why our cnostructs didn't work. Or we can test our repressors, promoters and inducers and have a systematic approach to anaylizing our system so that when something works or doesn't work we will know why. Thus we have designed a few tests which should give us relative and absolute values of our system that we can then plug into the model. | There are two methods we could follow in designing the Switch. We could randomly try different RBSs, hope it works and if not try again without having much of an understanding of why our cnostructs didn't work. Or we can test our repressors, promoters and inducers and have a systematic approach to anaylizing our system so that when something works or doesn't work we will know why. Thus we have designed a few tests which should give us relative and absolute values of our system that we can then plug into the model. | ||
| Line 182: | Line 267: | ||
We decided to to test for three values. The combined transcription/translation rate of each repressor, the cooperativity of each repressor and the concentration of ligand needed to deactivate each repressor. We managed to design three tests all using the same constructs so as to minimize ligations. These tests should determine our variables independantly, i.e. changing synthesis rate should not change cooperativity of repression. | We decided to to test for three values. The combined transcription/translation rate of each repressor, the cooperativity of each repressor and the concentration of ligand needed to deactivate each repressor. We managed to design three tests all using the same constructs so as to minimize ligations. These tests should determine our variables independantly, i.e. changing synthesis rate should not change cooperativity of repression. | ||
| - | + | ==Synthesis Rate== | |
The combined transcription/translation rate of the repressor is the combined strength of the promoter and the RBS. In [[Image:alphaTest.png|thumb|left|The Alpha test Architecture]]our model this is the alpha value. Our model predicts that the alpha values for each repressor should be fairly comparable (the stable region is along the 1 to 1 to 1 line in 3D). Since we can't change the promoter strength very easily, we will change the RBS strength to obtain similar alpha values for all repressors. | The combined transcription/translation rate of the repressor is the combined strength of the promoter and the RBS. In [[Image:alphaTest.png|thumb|left|The Alpha test Architecture]]our model this is the alpha value. Our model predicts that the alpha values for each repressor should be fairly comparable (the stable region is along the 1 to 1 to 1 line in 3D). Since we can't change the promoter strength very easily, we will change the RBS strength to obtain similar alpha values for all repressors. | ||
| Line 195: | Line 280: | ||
| - | + | ==Cooperativity of Repression== | |
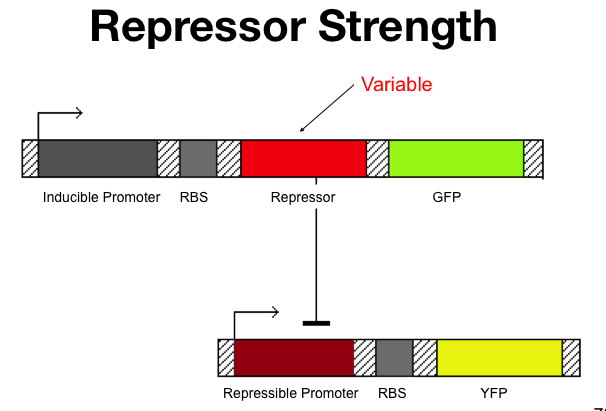
The cooperativity describes an inherent characteristic of a repressor's repression. In our system we want to know how [[Image:betaTest.png|thumb|left|The Beta test Architecture]]much increased repressor concentration will increase repression. In our model, cooperativity is an exponent, beta, so that the repressor concentration is raised to the beta ([repressor]^beta = total repression). For beta = 1, repression increases linearly with repressor concentration. With twice as much repressor there is twice the repression. For beta = 2, repression increases with the square of the concetration. Twce as much repressor leads to four times the repression. Our model predicts that our system will be more robust with greater alpha values and the tri stable region (in the graph) will be larger. Beta must be larger than one for the system to be stable. | The cooperativity describes an inherent characteristic of a repressor's repression. In our system we want to know how [[Image:betaTest.png|thumb|left|The Beta test Architecture]]much increased repressor concentration will increase repression. In our model, cooperativity is an exponent, beta, so that the repressor concentration is raised to the beta ([repressor]^beta = total repression). For beta = 1, repression increases linearly with repressor concentration. With twice as much repressor there is twice the repression. For beta = 2, repression increases with the square of the concetration. Twce as much repressor leads to four times the repression. Our model predicts that our system will be more robust with greater alpha values and the tri stable region (in the graph) will be larger. Beta must be larger than one for the system to be stable. | ||
| Line 202: | Line 287: | ||
| + | ==Ligand Concentration== | ||
| + | Naturally, we don't want to add more ligand than we need. In what ever application the project might find, if we wanted to change the state again by adding a different [[Image:ligandTest.png|thumb|left|The Ligand test Architecture]]ligand, we wouldn't want excessive amounts of the first ligand floating around. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| Line 222: | Line 304: | ||
| - | + | =Appendix= | |
| - | + | ==Schedule of Construction== | |
We have a schedule of ligations and transformations that we keep on googledocs to easily access information about the dna and cells in our freezer and fridge. This also organizes what ligations we plan to do in the future. In all there are about 40 ligations we want to do to construct the test constructs and first tristable system and based on the results of the tests, we will need to do as many as 20 more ligations. | We have a schedule of ligations and transformations that we keep on googledocs to easily access information about the dna and cells in our freezer and fridge. This also organizes what ligations we plan to do in the future. In all there are about 40 ligations we want to do to construct the test constructs and first tristable system and based on the results of the tests, we will need to do as many as 20 more ligations. | ||
| Line 245: | Line 327: | ||
| - | + | ==Other Predicted Obstacles== | |
#Other iGEM teams (info via Patrick King) have found that the araC gene contains a promoter region. Grown in TOP10 cells the construct araC>RBS>GFP>Terminator was observed to give a strong florescent output. In our system, this would give a florescent output for one FP even if that construct was not on. | #Other iGEM teams (info via Patrick King) have found that the araC gene contains a promoter region. Grown in TOP10 cells the construct araC>RBS>GFP>Terminator was observed to give a strong florescent output. In our system, this would give a florescent output for one FP even if that construct was not on. | ||
#AraC exhibits all or none gene expression in the presents of arabinose because the natural transporter, when induced, causes that cell to transport arabinose, depleting the culture. This may be overcome by adding a saturating level of arabinose. In the arabinose construct already made, we were not able to observe FP output even though there was a high level of arabinose. We used XL1Blue cells. | #AraC exhibits all or none gene expression in the presents of arabinose because the natural transporter, when induced, causes that cell to transport arabinose, depleting the culture. This may be overcome by adding a saturating level of arabinose. In the arabinose construct already made, we were not able to observe FP output even though there was a high level of arabinose. We used XL1Blue cells. | ||
#The degradation tags on the repressors may not give a long enough protein life time to yield strong repression. Thus, the tags may need to be removed to have a working device. | #The degradation tags on the repressors may not give a long enough protein life time to yield strong repression. Thus, the tags may need to be removed to have a working device. | ||
| - | + | ==Email Correspondances about Tri-Stable Switch== | |
[[Media:emailwithJasonLohmueller.txt]] | [[Media:emailwithJasonLohmueller.txt]] | ||
| Line 256: | Line 338: | ||
[[Media:emailwithPatrickKing.txt]] | [[Media:emailwithPatrickKing.txt]] | ||
| + | --> | ||
<html> | <html> | ||
</td> | </td> | ||
Latest revision as of 22:38, 25 October 2007

|
|||||||||||||||||