Imperial/Infector Detector/Specification
From 2007.igem.org
m |
|||
| (42 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Template:IC07navmenu}} | {{Template:IC07navmenu}} | ||
| - | + | <br clear="all"> | |
| + | <html> | ||
| + | <link rel="stylesheet" href="/igem07/index.php?title=User:Dirkvs/Stylesheets/IC07persist.css&action=raw&ctype=text/css" type="text/css" /> | ||
| + | |||
| + | <div id="tabs"> | ||
| + | <ul> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Introduction" title=""><span>Introduction</span></a></li> | ||
| + | <li><a class="current" href="https://2007.igem.org/Imperial/Infector_Detector/Specification" title=""><span>Specifications</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Design" title=""><span>Design</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Modelling" title=""><span>Modelling</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Implementation" title=""><span>Implementation</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Testing" title=""><span>Testing</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/F2620 Comparison" title=""><span>F2620</span></a></li> | ||
| + | <li><a href="https://2007.igem.org/Imperial/Infector_Detector/Conclusion" title=""><span>Conclusion</span></a></li> | ||
| + | </ul> | ||
| + | </div> | ||
| + | <hr /> | ||
| + | <br clear="all"> | ||
| + | </html> | ||
| + | __NOTOC__ | ||
= Infector Detector: Specifications = | = Infector Detector: Specifications = | ||
| - | + | The system must be able to detect the presence of biofilms on urinary catheters by detection of AHL, at a minimum concentration of 5nM, and report with a visual signal within 3 hours. It must work within a temperature range of 20°-30°C, be portable and easy to use, have a shelf life of at least seven days, and must not be harmful or infectious. | |
| - | < | + | |
| + | <!-- | ||
| + | |||
{|border="1" width="80%" align="center" | {|border="1" width="80%" align="center" | ||
|- | |- | ||
| Line 16: | Line 37: | ||
|style="background:#ffffcc"|Outputs | |style="background:#ffffcc"|Outputs | ||
|<center>System must give a visual signal if bacteria is present</center> | |<center>System must give a visual signal if bacteria is present</center> | ||
| + | |- | ||
| + | |style="background:#ffffcc"|Response Time | ||
| + | |<center>System needs to have a response time under 3 hour</center> | ||
|- | |- | ||
|style="background:#ffffcc"|Operating Conditions | |style="background:#ffffcc"|Operating Conditions | ||
|<center>System must operate within temperature 20-30°C</center> | |<center>System must operate within temperature 20-30°C</center> | ||
|- | |- | ||
| - | + | |style="background:#ffffcc"|Health & Safety | |
| - | + | |<center>System Must not be living replicating bacteria, and in any way harmful or infectious.</center> | |
| - | + | ||
| - | |style="background:#ffffcc"|Health | + | |
| - | |<center>System Must not be living replicating bacteria | + | |
| - | + | ||
| - | + | ||
| - | + | ||
|- | |- | ||
|style="background:#ffffcc"|Lifespan | |style="background:#ffffcc"|Lifespan | ||
| Line 37: | Line 55: | ||
<br clear="all"> | <br clear="all"> | ||
| + | == Specifications in detail == | ||
| - | == | + | --> |
| - | + | ||
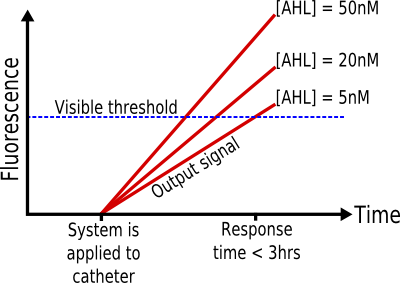
| + | [[Image:IC07 spec sysresponse.png|thumb|400px|left|'''Desired response''' of Infector Detector. Note that the response is not necessarily linear.]] | ||
| + | * '''<span style="color:#000077 ; font-size:120%;">Input:</span> AHL 5-50nM''' | ||
| + | It is known that, in Pseudomonas ''aeruginosa'' biofilms, the concentration of AHL is at least 5nM. <sup>[[#References|1-2]]</sup> Therefore, if the system can successfully report the presence of this concentration, it should be able to detect such biofilms. | ||
| + | * '''<span style="color:#000077 ; font-size:120%;">Output:</span> Visible fluorescent protein''' | ||
| + | In order for the system to be used by a nurse, without needing any special equipment, the output signal must be visible. | ||
| + | * '''<span style="color:#000077 ; font-size:120%;">Response Time:</span> < 3 hours''' | ||
| + | Given that biofilms grow and spread in a period of hours and days, the response time of the system must be as short as possible. The threshold of three hours is achievable in the time constraints of protein expression systems, yet it is short enough in comparison to the growth properties of biofilms. <sup>[[#References|3-6]]</sup> | ||
| + | * '''<span style="color:#000077 ; font-size:120%;">Operating conditions:</span> Temperatures from 20° to 30°C''' | ||
| + | The system will be applied to urinary catheters ''in-situ'', and therefore should function at the ambient temperature of hospitals - 20° to 30°C. | ||
| + | <br clear="all"> | ||
| + | * '''<span style="color:#000077 ; font-size:120%;">Health & safety:</span> Non-living, non-infectious''' | ||
| + | Because the system will be applied to urinary catheters ''in-situ'', it is likely that contact with the skin of the patient will occur. It is essential that it does not cause any harm or infection to the patient. Furthermore, any living organism is susceptible to mutations that may affect its intended function, or may proliferate in the environment of the catheter and urethra. | ||
| + | * '''<span style="color:#000077 ; font-size:120%;">Packaging and shelf-life:</span> 7 days''' | ||
| + | The system should be easily applied by a nurse caring for a patient with a urinary catheter. Hence, it should be packaged as a cream or a spray. In order to extend its usefulness and convenience, the system should also have a shelf-life of at least 7 days in a common hospital storage facility (shelves, cupboards, refrigerators, or freezers). | ||
| + | |||
| + | <br clear="all"> | ||
| - | + | <center> [https://2007.igem.org/Imperial/Infector_Detector/Introduction << Introduction] | Specifications | [https://2007.igem.org/Imperial/Infector_Detector/Design Design >>] | |
| + | </center> | ||
| - | + | == References == | |
| - | + | # Charlton, TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography–mass spectrometry: application to a model bacterial biofilm. Environmental Microbiology 2 (5), 530–541. October 2000. | |
| + | # Stickler DJ, Morris NS, McLean RJ, and Fuqua C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl Environ Microbiol 1998 Sep; 64(9) 3486-90. | ||
| + | # Morris NS, Stickler DJ, and McLean RJ. The development of bacterial biofilms on indwelling urethral catheters. World J Urol 1999 Dec; 17(6) 345-50. | ||
| + | # Drinka PJ. Complications of chronic indwelling urinary catheters. J Am Med Dir Assoc 2006 Jul; 7(6) 388-92. doi:10.1016/j.jamda.2006.01.020 pmid:16843240. | ||
| + | # Stickler, DJ, et al. A Sensor To Detect the Early Stages in the Development of Crystalline Proteus mirabilis Biofilm on Indwelling Bladder Catheters. Journal of Clinical Microbiology, April 2006, p. 1540-1542, Vol. 44, No. 4. | ||
| + | # [http://inls.ucsd.edu/~volfson/biofilm/ Growth and ordering of biofilms in controlled environments] available online on 22.10.2007 | ||
Latest revision as of 02:34, 27 October 2007

Infector Detector: Specifications
The system must be able to detect the presence of biofilms on urinary catheters by detection of AHL, at a minimum concentration of 5nM, and report with a visual signal within 3 hours. It must work within a temperature range of 20°-30°C, be portable and easy to use, have a shelf life of at least seven days, and must not be harmful or infectious.
- Input: AHL 5-50nM
It is known that, in Pseudomonas aeruginosa biofilms, the concentration of AHL is at least 5nM. 1-2 Therefore, if the system can successfully report the presence of this concentration, it should be able to detect such biofilms.
- Output: Visible fluorescent protein
In order for the system to be used by a nurse, without needing any special equipment, the output signal must be visible.
- Response Time: < 3 hours
Given that biofilms grow and spread in a period of hours and days, the response time of the system must be as short as possible. The threshold of three hours is achievable in the time constraints of protein expression systems, yet it is short enough in comparison to the growth properties of biofilms. 3-6
- Operating conditions: Temperatures from 20° to 30°C
The system will be applied to urinary catheters in-situ, and therefore should function at the ambient temperature of hospitals - 20° to 30°C.
- Health & safety: Non-living, non-infectious
Because the system will be applied to urinary catheters in-situ, it is likely that contact with the skin of the patient will occur. It is essential that it does not cause any harm or infection to the patient. Furthermore, any living organism is susceptible to mutations that may affect its intended function, or may proliferate in the environment of the catheter and urethra.
- Packaging and shelf-life: 7 days
The system should be easily applied by a nurse caring for a patient with a urinary catheter. Hence, it should be packaged as a cream or a spray. In order to extend its usefulness and convenience, the system should also have a shelf-life of at least 7 days in a common hospital storage facility (shelves, cupboards, refrigerators, or freezers).
References
- Charlton, TS, et al. A novel and sensitive method for the quantification of N-3-oxoacyl homoserine lactones using gas chromatography–mass spectrometry: application to a model bacterial biofilm. Environmental Microbiology 2 (5), 530–541. October 2000.
- Stickler DJ, Morris NS, McLean RJ, and Fuqua C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl Environ Microbiol 1998 Sep; 64(9) 3486-90.
- Morris NS, Stickler DJ, and McLean RJ. The development of bacterial biofilms on indwelling urethral catheters. World J Urol 1999 Dec; 17(6) 345-50.
- Drinka PJ. Complications of chronic indwelling urinary catheters. J Am Med Dir Assoc 2006 Jul; 7(6) 388-92. doi:10.1016/j.jamda.2006.01.020 pmid:16843240.
- Stickler, DJ, et al. A Sensor To Detect the Early Stages in the Development of Crystalline Proteus mirabilis Biofilm on Indwelling Bladder Catheters. Journal of Clinical Microbiology, April 2006, p. 1540-1542, Vol. 44, No. 4.
- [http://inls.ucsd.edu/~volfson/biofilm/ Growth and ordering of biofilms in controlled environments] available online on 22.10.2007