BerkiGEM2007Present3
From 2007.igem.org
JCAnderson (Talk | contribs) (→Design and construction) |
m |
||
| (47 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| + | <html> | ||
| + | <style> | ||
| + | <!-- | ||
| + | |||
| + | #apDiv3 { | ||
| + | position:absolute; | ||
| + | left:10px; | ||
| + | top:-108px; | ||
| + | width:101px; | ||
| + | height:87px; | ||
| + | z-index:900; | ||
| + | } | ||
| + | |||
| + | #apDiv1 { | ||
| + | position:absolute; | ||
| + | left:2.5%; | ||
| + | top:50px; | ||
| + | width:95%; | ||
| + | height:90%; | ||
| + | z-index:1; | ||
| + | background-color: #FFFFFF; | ||
| + | } | ||
| + | body { | ||
| + | background-image: url(); | ||
| + | background-color: #FFFFFF; | ||
| + | background-repeat: repeat; | ||
| + | text-align: justify; | ||
| + | } | ||
| + | a:link { | ||
| + | color: #FF0000; | ||
| + | } | ||
| + | a:visited { | ||
| + | color: #990000; | ||
| + | } | ||
| + | a:hover { | ||
| + | color: #FF0000; | ||
| + | } | ||
| + | a:active { | ||
| + | color: #FF0000; | ||
| + | } | ||
| + | h1,h2,h3,h4,h5,h6 { | ||
| + | font-weight: bold; | ||
| + | } | ||
| + | h1 { | ||
| + | font-size: xx-large; | ||
| + | color: #000000; | ||
| + | text-align: center; | ||
| + | } | ||
| + | .firstHeading { | ||
| + | text-align: left; | ||
| + | } | ||
| + | h2 { | ||
| + | font-size: x-large; | ||
| + | color: #000000; | ||
| + | text-align: center; | ||
| + | } | ||
| + | h3 { | ||
| + | font-size: large; | ||
| + | color: #000000; | ||
| + | } | ||
| + | --> | ||
| + | </style> | ||
| + | <body> | ||
| + | <div id="apDiv3"><a href="https://2007.igem.org/Main_Page"><img name="Coverlogo" src="https://static.igem.org/mediawiki/2007/0/0e/Coverlogojpeg.jpg" width="101" height="87" alt=""></div></a> | ||
| + | </body> | ||
| + | </html> | ||
__NOTOC__ | __NOTOC__ | ||
| - | + | <html> | |
| + | <link rel="stylesheet" href="http://www.davidtulga.com/controller.css" type="text/css" /> | ||
| + | <script type="text/javascript" src="http://www.davidtulga.com/controller.js"></script> | ||
| + | <div align="center"> | ||
| + | <p><a href="https://2007.igem.org/Berkeley_UC"><<< Return to UC Berkeley iGEM 2007 </a></p> | ||
| + | <p> <a href="https://2007.igem.org/BerkiGEM2007Present4"><<Previous Section: Chassis</a> | <a href="https://2007.igem.org/BerkiGEM2007Present5">Next Section: Genetic Self-Destruct>></a></p> | ||
| + | </div></html> | ||
| - | + | =The Controller= | |
| - | The Controller is an integrated genetic circuit, comprised of two plasmids, that directs the | + | The Controller is an integrated genetic circuit, comprised of two plasmids, that directs the copy number and transcription of the primary devices in our system. |
| - | + | ==Introduction== | |
| - | The Bactoblood organism needs to exist in two different states: one form that is genetically stable and able to grow under normal laboratory conditions, and a second state that is highly differentiated, unable to grow, and devoid of genetic material. To bring about | + | The Bactoblood organism needs to exist in two different states: one form that is genetically stable and able to grow under normal laboratory conditions, and a second state that is highly differentiated, unable to grow, and devoid of genetic material. To bring about the transformation to the differentiated state, we needed a controller that could be easily triggered by an external cue. This controller required a large dynamic range between the off and on states, the ability to maintain and overexpress a large number of genes, and ideally employed a low-cost inducer. Therefore, we designed a controller based on a two plasmid system. One plasmid stably replicates the various biosynthetic operons of our system at single copy in a transcriptionally-inactive state. The second plasmid houses the genes necessary for activation of the operon plasmid. When activated with iron, the copy number of the operon plasmid increases to high-copy and the transcription of the operons is activated. |
| - | + | ==Design and construction== | |
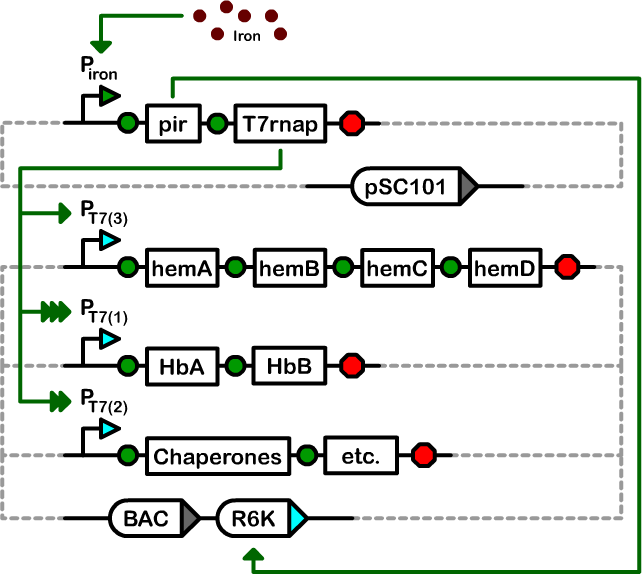
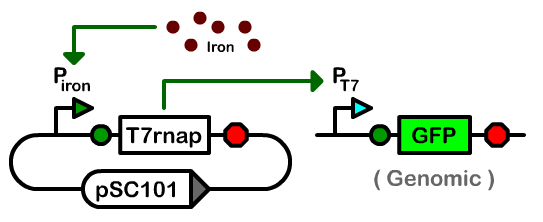
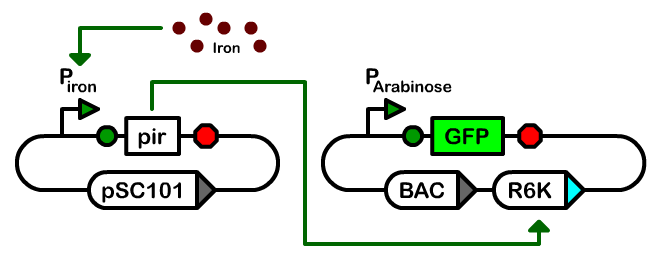
| - | + | We designed a two-plasmid architecture in which the biosynthetic operons reside on a single-copy bacterial artificial chromosome (BAC). The operons are under the transcriptional control of T7 promoters of various strengths. The BAC also contains an R6K origin of replication. In most strains of ''E. coli'', this origin is silent as it requires the expression of the ''pir'' gene for replication. The second plasmid in our controller is a low-copy pSC101-derived plasmid that houses the T7 RNA polymerase and ''pir'' genes under the control of an iron-inducible promoter.<br><br> | |
| + | [[Image:Berk-DT Figure 1.png|575px]] | ||
| + | [[Image:Berk-DT Figure Legend.png|325px]]<br><br> | ||
| - | [[Image: | + | ===Construction of an iron-responsive PoPS-generating device=== |
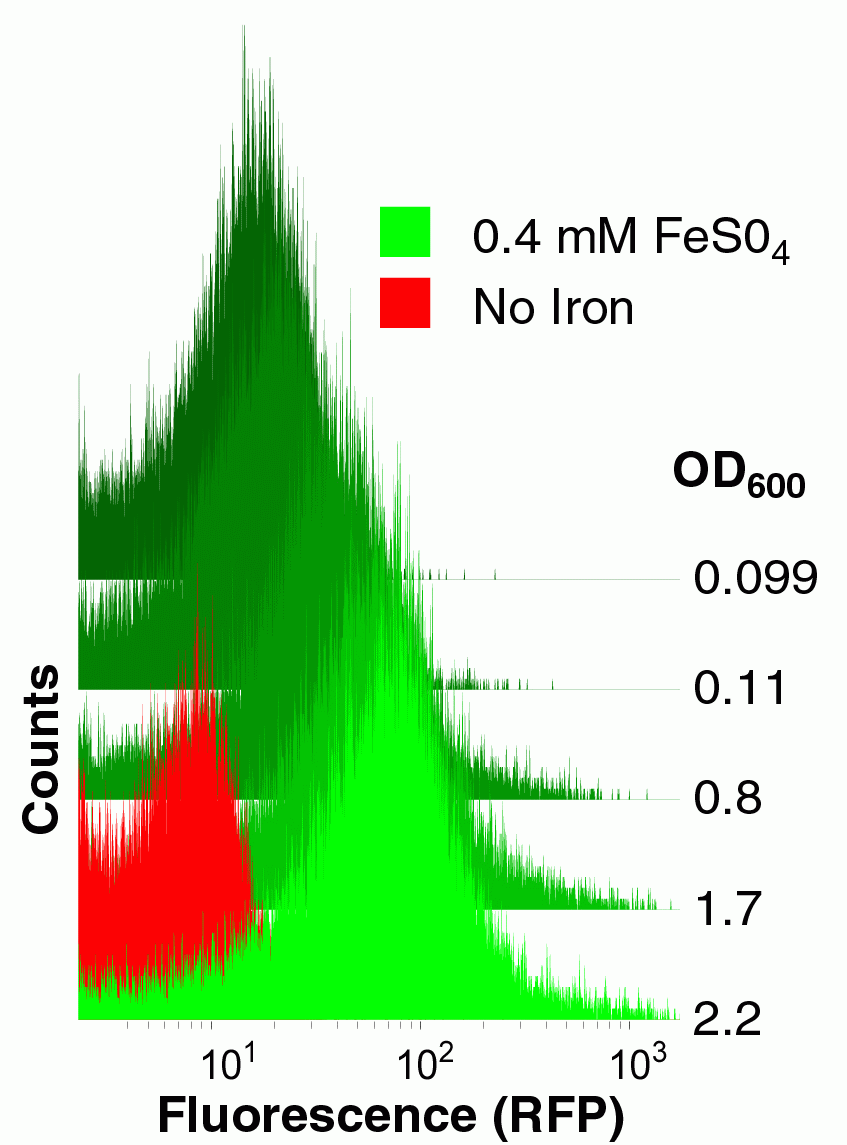
| + | [[Image:BerkiGEM2007-yfbEcytometry.jpg|thumb|200px|right|'''An iron-inducible promoter.''' Cells were transformed with an RFP transcriptional reporter device derived from our yfbE promoter part and grown with or without exogenous iron to various densities and then analyzed for fluorescence by cytometry.]] | ||
| - | [[Image: | + | To construct this system, first we needed a promoter that was induced by iron. Microarray studies suggested that the ''yfbE'' promoter of ''E. coli'' might function as an iron-responsive PoPS-generating device. We therefore constructed a Biobrick derived from the ''yfbE'' promoter and constructed an RFP reporter composite part derived from this basic part. We examined the fluorescence of cells harboring this part both as a function of external iron concentration and growth phase. The ''yfbE'' promoter part had the ideal qualities for our controller: it is induced 100-fold as the bacteria emerge from the mid-log phase of growth, but only in the presence of exogenous iron. |
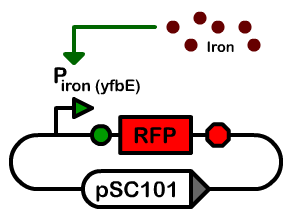
| + | [[Image:BerkiGEM2007-Figure-Piron-vector.png|thumb|left|200px|'''Vectorology of the iron promoter characterization construct''']] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br> | ||
| - | === | + | ===Construction of an iron-dependent transcription device=== |
| + | To control gene expression we needed to place the T7 RNA polymerase under the control of the yfbE promoter on a pSC101-derived plasmid. We therefore made a T7 RNA polymerase basic part and constructed a library of composite parts containing the yfbE part, one of nine ribosome binding site parts of different strengths, and the T7 RNA polymerase gene with a GTG or an ATG start codon. We constructed these composite parts in the pSC101 Biobrick plasmid I716101 and then examined their activity in an engineered ''E. coli'' strain, GH455G, containing a genome-integrated cassette with GFP under the control of a T7 promoter. Of the composite parts we constructed, only the composite part with the weakest ribosome binding site and a GTG start codon showed iron-dependent GFP production. All composite parts with an ATG start were too active and toxic, while the other ribosome binding sites were either constitutively on or off. | ||
| + | [[Image:BerkiGEM2007-Figure-T7-vector.png|thumb|left|367px|'''Vectorology of the T7 RNA Polymerase characterization construct''']] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br> | ||
| - | + | ===Construction of an iron-dependent copy number device=== | |
| + | [[Image:BerkiGEM2007-PirCytometry.jpg|thumb|200px|left|'''Iron-inducible Copy Number.''' Cells containing an optimized ''yfbE-pir'' controller, a ''pir116'' strain, and a non-pir strain were transformed with an inducible BAC plasmid encoding GFP. As the copy number increases, so does the amount of GFP produced. Only the controller cells show iron-dependent copy number.]] | ||
| + | [[Image:BerkiGEM2007-YfbEPirScreen.jpg|375px]]<br> | ||
| + | The third component of our controller was an iron-inducible copy number amplifier system. We therefore constructed a ''pir'' basic part and attempted to make composite parts with the ''yfbE'' promoter part. We were unable to construct Biobrick-derived cassettes of this composition apparently due to toxicity. Instead, we constructed a ribosome binding site-free version of the part in the pSC101-derived plasmid. We subsequently introduced a library of ribosome binding sites by EIPCR in which the start codon was either ATG or GTG and positions 11-13 upstream of start codon were randomized. We introduced the library plasmids into ''E. coli'' cells harboring plasmid pBACr-AraGFP which is a BAC plasmid with an R6K origin of replication and GFP under an arabinose-inducible promoter. Due to the lack of a pir gene in these cells, only a low level of GFP production is observed when they are grown in arabinose media. In contrast, the production of GFP in cells with an induced pir gene is 200-fold greater, similar to the GFP production in the pir116 strain. | ||
| + | [[Image:BerkiGEM2007-Figure-pir-vector.png|thumb|left|400px|'''Vectorology of the ''pir'' characterization construct''']] | ||
| + | <br><br><br><br><br><br><br><br><br><br><br> | ||
| - | + | ==Conclusion== | |
| - | + | ||
| - | ==== | + | |
| - | + | ||
| - | + | Our controller system allows for a dramatic induction of the operons present on our BAC upon the introduction of free iron into the system. It accomplishes this by utilizing both the ''pir'' gene to amplify the R6K origin, as well as the T7 RNA polymerase transcription system. This provides not only excellent dynamic range, but also the ability to tune the relative expression levels of the operons under T7 promoter control. These capabilities allow the Bactoblood organism to adopt its two distinct phenotypes required for normal growth in the laboratory, and static oxygen carrying in the bloodstream. | |
| - | + | ||
| - | === | + | <html> |
| - | + | <p align="justify"> </p> | |
| + | <p align="center"><a href="https://2007.igem.org/Berkeley_UC"><<< Return to UC Berkeley iGEM 2007 </a></p> | ||
| + | <p align="center"> <a href="https://2007.igem.org/BerkiGEM2007Present4"><<Previous Section: Chassis</a> | <a href="https://2007.igem.org/BerkiGEM2007Present5">Next Section: Genetic Self-Destruct>></a></p> | ||
| + | </html> | ||
Latest revision as of 04:10, 27 October 2007
<<< Return to UC Berkeley iGEM 2007
<<Previous Section: Chassis | Next Section: Genetic Self-Destruct>>
The Controller
The Controller is an integrated genetic circuit, comprised of two plasmids, that directs the copy number and transcription of the primary devices in our system.
Introduction
The Bactoblood organism needs to exist in two different states: one form that is genetically stable and able to grow under normal laboratory conditions, and a second state that is highly differentiated, unable to grow, and devoid of genetic material. To bring about the transformation to the differentiated state, we needed a controller that could be easily triggered by an external cue. This controller required a large dynamic range between the off and on states, the ability to maintain and overexpress a large number of genes, and ideally employed a low-cost inducer. Therefore, we designed a controller based on a two plasmid system. One plasmid stably replicates the various biosynthetic operons of our system at single copy in a transcriptionally-inactive state. The second plasmid houses the genes necessary for activation of the operon plasmid. When activated with iron, the copy number of the operon plasmid increases to high-copy and the transcription of the operons is activated.
Design and construction
We designed a two-plasmid architecture in which the biosynthetic operons reside on a single-copy bacterial artificial chromosome (BAC). The operons are under the transcriptional control of T7 promoters of various strengths. The BAC also contains an R6K origin of replication. In most strains of E. coli, this origin is silent as it requires the expression of the pir gene for replication. The second plasmid in our controller is a low-copy pSC101-derived plasmid that houses the T7 RNA polymerase and pir genes under the control of an iron-inducible promoter.
Construction of an iron-responsive PoPS-generating device
To construct this system, first we needed a promoter that was induced by iron. Microarray studies suggested that the yfbE promoter of E. coli might function as an iron-responsive PoPS-generating device. We therefore constructed a Biobrick derived from the yfbE promoter and constructed an RFP reporter composite part derived from this basic part. We examined the fluorescence of cells harboring this part both as a function of external iron concentration and growth phase. The yfbE promoter part had the ideal qualities for our controller: it is induced 100-fold as the bacteria emerge from the mid-log phase of growth, but only in the presence of exogenous iron.
Construction of an iron-dependent transcription device
To control gene expression we needed to place the T7 RNA polymerase under the control of the yfbE promoter on a pSC101-derived plasmid. We therefore made a T7 RNA polymerase basic part and constructed a library of composite parts containing the yfbE part, one of nine ribosome binding site parts of different strengths, and the T7 RNA polymerase gene with a GTG or an ATG start codon. We constructed these composite parts in the pSC101 Biobrick plasmid I716101 and then examined their activity in an engineered E. coli strain, GH455G, containing a genome-integrated cassette with GFP under the control of a T7 promoter. Of the composite parts we constructed, only the composite part with the weakest ribosome binding site and a GTG start codon showed iron-dependent GFP production. All composite parts with an ATG start were too active and toxic, while the other ribosome binding sites were either constitutively on or off.
Construction of an iron-dependent copy number device
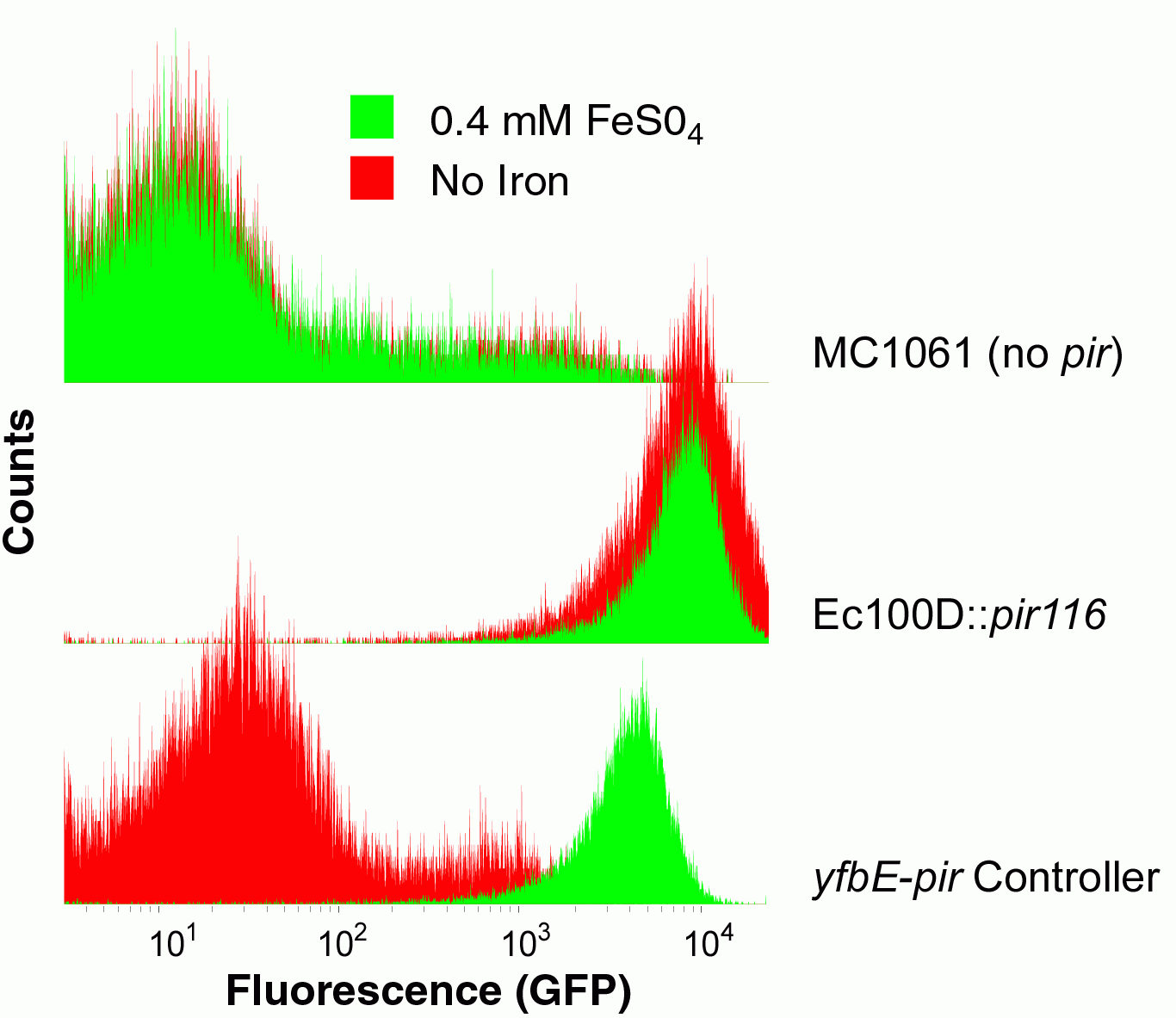
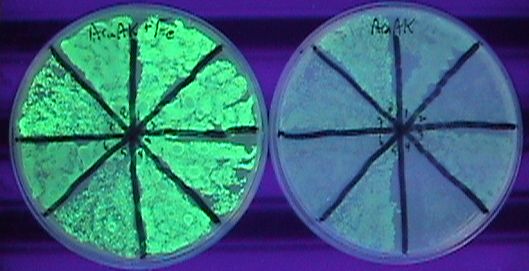
The third component of our controller was an iron-inducible copy number amplifier system. We therefore constructed a pir basic part and attempted to make composite parts with the yfbE promoter part. We were unable to construct Biobrick-derived cassettes of this composition apparently due to toxicity. Instead, we constructed a ribosome binding site-free version of the part in the pSC101-derived plasmid. We subsequently introduced a library of ribosome binding sites by EIPCR in which the start codon was either ATG or GTG and positions 11-13 upstream of start codon were randomized. We introduced the library plasmids into E. coli cells harboring plasmid pBACr-AraGFP which is a BAC plasmid with an R6K origin of replication and GFP under an arabinose-inducible promoter. Due to the lack of a pir gene in these cells, only a low level of GFP production is observed when they are grown in arabinose media. In contrast, the production of GFP in cells with an induced pir gene is 200-fold greater, similar to the GFP production in the pir116 strain.
Conclusion
Our controller system allows for a dramatic induction of the operons present on our BAC upon the introduction of free iron into the system. It accomplishes this by utilizing both the pir gene to amplify the R6K origin, as well as the T7 RNA polymerase transcription system. This provides not only excellent dynamic range, but also the ability to tune the relative expression levels of the operons under T7 promoter control. These capabilities allow the Bactoblood organism to adopt its two distinct phenotypes required for normal growth in the laboratory, and static oxygen carrying in the bloodstream.
<<< Return to UC Berkeley iGEM 2007
<<Previous Section: Chassis | Next Section: Genetic Self-Destruct>>