Chiba/Goal
From 2007.igem.org
(→A test of size control) |
(→A test to form Marimo) |
||
| Line 65: | Line 65: | ||
If succeeded,AHL from senders spreads.<br> | If succeeded,AHL from senders spreads.<br> | ||
Receivers express GFP and FliC-His,and besides,bond with cobalt ions. | Receivers express GFP and FliC-His,and besides,bond with cobalt ions. | ||
| - | <br>Finnally,form marimos.<br><br><br><br><br><br><br><br> | + | <br>Finnally,form marimos.<br><br><br><br><br><br><br><br><br><br> |
===A test of size control=== | ===A test of size control=== | ||
Revision as of 05:25, 27 October 2007
|
Home |
Our Goal
As Final-Construction, we will carry out four experiments.
- A test of adsorption between flagella
- A test to confirm a limit of size
- A test to form Marimo
- A test of size control
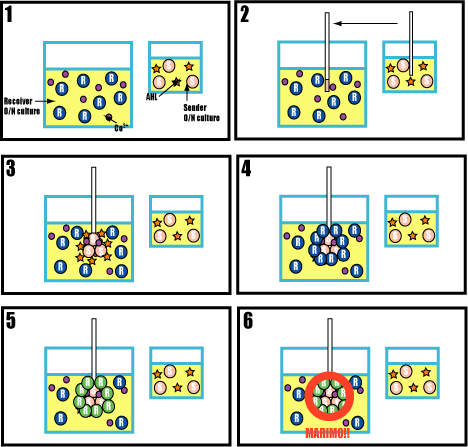
A test of adsorption between flagella
Purpose
Confirm adsorption between senders and receivers with His-Tag.
Method
- Culture senders and receivers in a test tube.
- Drop senders into receivers tube and mix cobalt ion.
- Divide it into two groups. One is added imidazole and the other is non imidazole. Inoculate them on the plates.
- Check the colony.
Prediction
If succeeded, cultures which is added imidazole will form a indipendent colony of senders&receivers. The other (non imidazole) will form colonies of adsorbed senders and receivers.
A test to confirm a limit of size
Purpose
The purpose of this test is to confirm that Marimo has a limit of size on a plate.
Method
- Culture senders and receivers in a test tube.
- Inoculate receivers equally on the plate, and drop senders at the center of it.
- After over night,pick colonies which are near the senders (colonies express GFP), the end of the circle expressed GFP, and out of the green circle.
- Dilute them.
- Mix cobalt ion into dilutions and inoculate on the plates.
- Check the colony.
Prediction
If succeeded,receivers which don’t express FliC (they didn’t sense AHL and express GFP) form colonies one by one.
And receivers expressed FliC (they sensed AHL and expressed GFP) form aggregated colonies.)
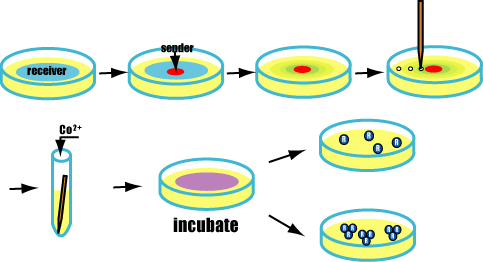
A test to form Marimo
Purpose
Actually we make Marimo.
- AHL diffuse fast because it is very small molecule.
- Packed senders into a very thin capillary . AHL from it diffuse slowly and form concentrate gradients. AHL gradients enable Marimos to aggregate like a real ball.
Method
- Culture senders and receivers in each test tube and mix Cobalt ions in receiver's tube.
- Add senders to receivers with a capillary.
- Check Marimos.
Prediction
If succeeded,AHL from senders spreads.
Receivers express GFP and FliC-His,and besides,bond with cobalt ions.
Finnally,form marimos.
A test of size control
Purpose
Control marimo's size by changing the ratio of amount of senders and receivers.
Method
- Culture senders and receivers in each test tube.
- Observe marimo's size by changing the amount of receivers and their types.(const.senders)
- Observe marimo's size by changing the amount of senders.(const.receivers)
Prediction
Low concentration of senders or receivers : Mini Marimo
High concentration of senders or receivers : Big Marimo
Advanced Goal in Our Future
- Make Marimo which cross section is colorful. Receivers form layers and express various fluorescent proteins. When we cut Marimo, We will be able to observe beautiful gradation.
- Bacteria Marimo grows like Real Marimo!
Bacteria Marimo grows when it is lighted as if performing photosynthesis like natural Marimo.
This gene circuit contains light sensor gene. LuxI represses light senser.
- Prepare mixture of Senders, Receivers(AHL -> FliC-His and GFP), and Co2+.
- Senders express FliC-His but do not synthesize AHL. Senders aggregate FliC-His and Co ions.
- When the culture is lighted, senders express LuxI and synthesize AHL.
- Receivers express FliC-His and GFP.
- Receivers aggregate around the Senders core.
Other than that, we have many FUTURE IDEAS (very far future...far enough we can leave CHIBA University happy) such as Marimo-mediated polous materials/ gels. Also presented are the other ways to make marimo in completely different strategies. To check those out, please visit our [http://chem.tf.chiba-u.jp/igem/ official website] (keep updated; never get frozen)