Naples
From 2007.igem.org
| Line 52: | Line 52: | ||
Another important assumption is the following: | Another important assumption is the following: | ||
*Transcription factor timescales are much larger than protein-protein interactions timescales | *Transcription factor timescales are much larger than protein-protein interactions timescales | ||
| + | *Input changes are very rapid | ||
We will use this assumption to simplify our modeling | We will use this assumption to simplify our modeling | ||
| Line 64: | Line 65: | ||
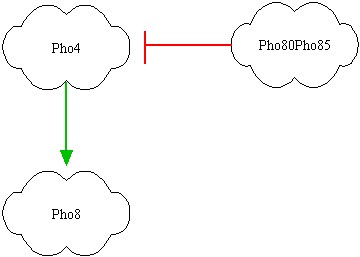
In the left picture the genes are represented; red link indicates a protein-protein interactions, while green link indicates a transcription factor interaction. It's clear that Pho80Pho85 inhibits Pho4, so il Pho80Pho85 is active then Pho8 is low even if Pho4 is active (design task). If Pho80Pho85 is inactive and Pho4 is active, then Pho8 becomes active. If Pho80Pho85 and Pho4 are inactive, than Pho8 is inactive. The inhibition of Pho4 by Pho80Pho85 is at protein-protein level: if Pho80Pho85 is active it phosphoriles Pho4 so that the Pho4 concentration becomes rapidly small and thus it can't activate Pho8. | In the left picture the genes are represented; red link indicates a protein-protein interactions, while green link indicates a transcription factor interaction. It's clear that Pho80Pho85 inhibits Pho4, so il Pho80Pho85 is active then Pho8 is low even if Pho4 is active (design task). If Pho80Pho85 is inactive and Pho4 is active, then Pho8 becomes active. If Pho80Pho85 and Pho4 are inactive, than Pho8 is inactive. The inhibition of Pho4 by Pho80Pho85 is at protein-protein level: if Pho80Pho85 is active it phosphoriles Pho4 so that the Pho4 concentration becomes rapidly small and thus it can't activate Pho8. | ||
The ''outputs'' of the system will be Pho80Pho85 and Pho8 and we will associate them ''red'' or ''yellow'' lights! | The ''outputs'' of the system will be Pho80Pho85 and Pho8 and we will associate them ''red'' or ''yellow'' lights! | ||
| + | |||
| + | ==== Mathematical model ==== | ||
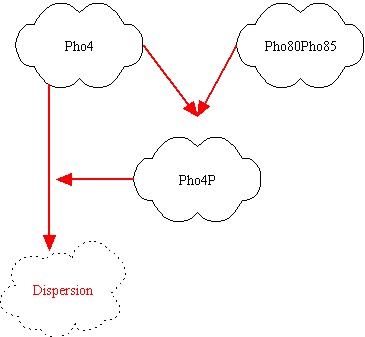
| + | We modeled the transcriptions factor interactions as Hill-like functions, while the protein-protein interactions are represented through a typical ''prey-predator'' model. | ||
| + | For the transcription factor level we have the following equations: | ||
== Yeast Strain == | == Yeast Strain == | ||
Revision as of 10:37, 19 July 2007
Contents |
University of Naples "Federico II"
Tigem
The Telethon Institute of Genetics and Medicine (TIGEM) was created by the Italian Telethon Foundation in 1994. TIGEM mission is the understanding of the pathogenic mechanisms of genetic diseases with the aim of developing preventive and therapeutic strategies.TIGEM currently hosts 17 research groups, and a total of more than 120 persons, including students, postdoctoral fellows, staff scientists, technicians, and administrators and offers training programs in medical human genetics and Synthetic Biology in cooperation with the University of Naples Federico II.
- Students:
- Giovanni Russo
- Lucia Marucci
- Velia Siciliano
- Irene Cantone
- Roberta Bergamasco
- Maria Aurelia Ricci
- Mafalda Graziano
- Instructors
- Diego di Bernardo
- Maria Pia Cosma
- Mario di Bernardo
- Advisor
- Giulia Cuccato
Our Project
The aim of our project is to engineer a synthetic biological network and modifying Saccharomyces cerevisiae cells so that we would be able to change colour at differents oleate concentrations.
Oleate is the principal olive oil element and acidity indicator. The olive oil is defined "extra vergine" if it has an acidity lower than 0.8 %,"vergine" with an acidity lower than 2% and not commestible if has an acidity higher than 3%. Oleate induces the transcription of genes involved in peroxisome biogenesis and stimulates the proliferation of these organelles in Saccharomyces cerevisiae. Fatty acid-mediated induction is based on a dramatic increase in transcription of several genes encoding peroxisomal functions due to the presence of an oleate response element (ORE) in their promoters.This upstream activating sequence is minimally defined by an inverted repeat of CGG triplets separated by a 15-18-nucleotide spacer. It constitutes the binding target for the transcription factors Oaf1p and Pip2p.
Modeling
Introduction
Over the past decades progress in measurement of rates and interactions of molecular and cellular processes has initiated a revolution in understanding of dynamical phenomena in cells. Generally speaking a dynamical phenomenon is a process that changes over time. Living cells are inherently dynamic! Indeed, to sustain the characteristic features of life (growth, cell division...) they need to axtract and trasform energy from their surroundings. This implies that cells function thermodinamically as open systems. So, we have encountered a new keyword: system. The most general definition for system is the following: a set of functional elements joint together to perform a specific task. Cells are astoundingly complex systems: they contain networks of thousands of biochemical interactions. System-level understanding, the approach of systems biology, requires a shift in the notion of what to look for. An understanding of genes and proteins is very important, but now the focus is on understanding system' structure and dynamics. Biologists use cartoons ro capture the complexity of the networks, but because a system is not just an assembly of genes and proteins, its properties cannot be fully understood by drawing these diagrams. They, of course, represent a first step in our modeling, but can be compared to static roadmap, whereas what we really seek to know are the traffic patterns, why they energe, how to control them. So, we will use a typical approach from systems and control theory.
Basic assumptions
We realized a reaction network as a system of ODEs. Clearly we need to guess working hypothesis:
- Component concentrations don't vary with respect to time
Whether it is a good assumption depends on time and space scales. In a yeast cell molecular diffution is sufficiently fast to mix proteins in less than one minute
- Component concentrations are continuos functions of time
This is true if the number of molecules of each species in the reaction volume is sufficiently large
Another important assumption is the following:
- Transcription factor timescales are much larger than protein-protein interactions timescales
- Input changes are very rapid
We will use this assumption to simplify our modeling
ODEs are very useful to represent molecular interaction. Indeed, applying a simple set of rules we can represent arbitrarily complex reaction networks as a set of coupled ODEs!
Our model
After some brainstorming we found the best parts for our project. They are showed above:
Notice that our network will respond to medium oleate concentrations (vergine oil) and to high oleate concentrations (non commestible oil), while for small oleate concentrations (extra vergine oil) it doesn't respond. In the left picture the genes are represented; red link indicates a protein-protein interactions, while green link indicates a transcription factor interaction. It's clear that Pho80Pho85 inhibits Pho4, so il Pho80Pho85 is active then Pho8 is low even if Pho4 is active (design task). If Pho80Pho85 is inactive and Pho4 is active, then Pho8 becomes active. If Pho80Pho85 and Pho4 are inactive, than Pho8 is inactive. The inhibition of Pho4 by Pho80Pho85 is at protein-protein level: if Pho80Pho85 is active it phosphoriles Pho4 so that the Pho4 concentration becomes rapidly small and thus it can't activate Pho8. The outputs of the system will be Pho80Pho85 and Pho8 and we will associate them red or yellow lights!
Mathematical model
We modeled the transcriptions factor interactions as Hill-like functions, while the protein-protein interactions are represented through a typical prey-predator model. For the transcription factor level we have the following equations:
Yeast Strain
Yeast strain used is W303. All DNA manipulations and subcloning were done in Escherichia coli.
Materials & Methods
We have adopted a strategy of parallel cloning .Two different reporter genes (luciferase and B-galattosidasy)are cloned in parallel into a vector containing four different promoters.
- Cloning Strategies
- Vector choice
- Restriction Enzyme
- Primers Design