Melbourne/Blue Photosensor
From 2007.igem.org
m |
|||
| (11 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
[[Melb:Background|<return to top of background>]] [[melbourne|<return to home page>]] [[Melb:And Gate |<next>]] | [[Melb:Background|<return to top of background>]] [[melbourne|<return to home page>]] [[Melb:And Gate |<next>]] | ||
| + | As part of the overall system design, a blue light sensitive pathway is required in addition to the red light sensitive pathway. Described below is the blue photosensor. This involves the design of a chimeric trans-membrane protein. A blue light sensitive (~500nm) integral photo receptor SopII that dimerizes with a histidine kinase; HtrII (As described in 2001 paper). | ||
| + | The kinase domain of HtrII will be replaced with the kinase domain of ComP. ComP forms part of a two-component system from Bacillus subtilis - and this will not affect any endogenous networks in e.coli. | ||
| + | The two component system involves: ComP, a two-component sensor histidine kinase and ComA, a two-component response regulator. Phosphorylated comA will upregulate transcription at the psfA/srfA promoter [[Melbourne/Lab_BL_Notebook/PsrfA_sequence|psfA/srfA]], as part of the AND gate. | ||
| + | |||
| + | '''In summary''': | ||
| + | |||
| + | [[Image:Melbourne SrII-HtrII.JPG|200px|SrII-HtrII]] + [[Image:Melbourne ComP.JPG|200px|ComP]] >>>>>> [[Image:Melbourne HtrII-ComPchimera.jpg|200px|HtrII-ComP fusion]] | ||
====[[Melbourne/Blue Photosensor Background|Blue Photosensor Background]]==== | ====[[Melbourne/Blue Photosensor Background|Blue Photosensor Background]]==== | ||
| Line 7: | Line 14: | ||
====[[Melbourne/Plan/Blue Photosensor|Method]] ==== | ====[[Melbourne/Plan/Blue Photosensor|Method]] ==== | ||
| + | ====Possible extensions:==== | ||
| + | *Determination of optimal wavelength: | ||
| + | **use of different substrates (different retinals) | ||
| + | *Separate variants all submitted as BioBricks. | ||
| + | *Submitted synthesized ComP and ComA as BioBricks | ||
| + | *Model the pathway to determine rate-limiting step | ||
| + | |||
| + | ====References==== | ||
This part is based on “Photostimulation of a Sensory Rhodopsin II/HtrII/Tsr Fusion Chimera Activates CheA-Autophosphorylation and CheY-Phosphotransfer in Vitro” by Vishwa D. Trivedi and John L. Spudich, Biochemistry 2003, 42, 13887-13892. | This part is based on “Photostimulation of a Sensory Rhodopsin II/HtrII/Tsr Fusion Chimera Activates CheA-Autophosphorylation and CheY-Phosphotransfer in Vitro” by Vishwa D. Trivedi and John L. Spudich, Biochemistry 2003, 42, 13887-13892. | ||
| - | Acording to this article the peak sensitivity is to 500+/-5nm, and results in a 3 fold activation of the Tsr | + | Acording to this article the peak sensitivity is to 500+/-5nm, and results in a 3 fold activation of the Tsr (wild type). CheA,W,Y connected system. |
| - | It is proposed to replace Tsr with homolgouse | + | It is proposed to replace Tsr fusion with homolgouse ComP. |
| - | SRII-HtrII fusion to which | + | SRII-HtrII fusion to which ComP is fused |
| - | + | ComA when phosphorylated by ComP is an activator for PsfA promoter sequence from | |
Dr Alan Grossman (M.I.T.) | Dr Alan Grossman (M.I.T.) | ||
Based on | Based on | ||
| Line 28: | Line 43: | ||
SRII is from Natronomonas pharaonis. | SRII is from Natronomonas pharaonis. | ||
| - | Tsr fusion was made by Jung et al J Bacteriol 183 6365-6371 (2001) they propose a mechanism | + | Tsr fusion was made by Jung et al J Bacteriol 183 6365-6371 (2001) they propose a mechanism. |
| - | + | ||
| - | + | ||
Latest revision as of 13:44, 26 October 2007
<return to top of background> <return to home page> <next>
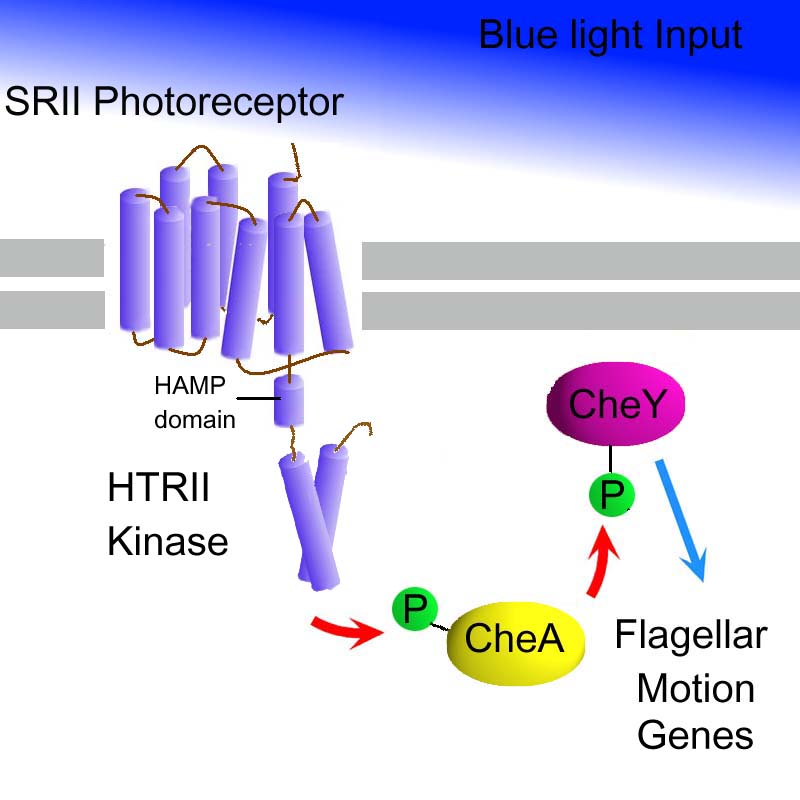
As part of the overall system design, a blue light sensitive pathway is required in addition to the red light sensitive pathway. Described below is the blue photosensor. This involves the design of a chimeric trans-membrane protein. A blue light sensitive (~500nm) integral photo receptor SopII that dimerizes with a histidine kinase; HtrII (As described in 2001 paper).
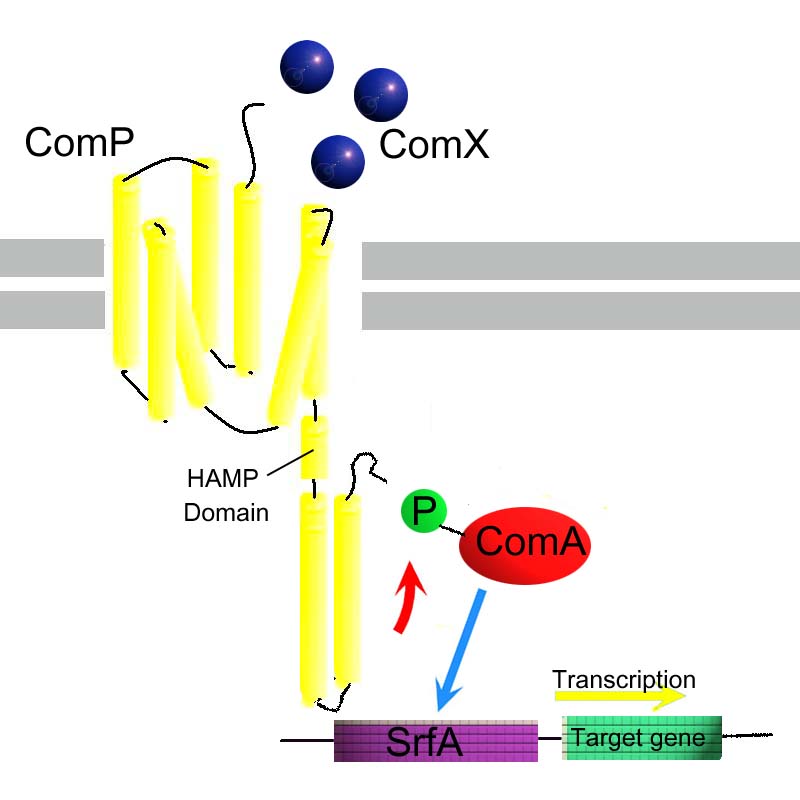
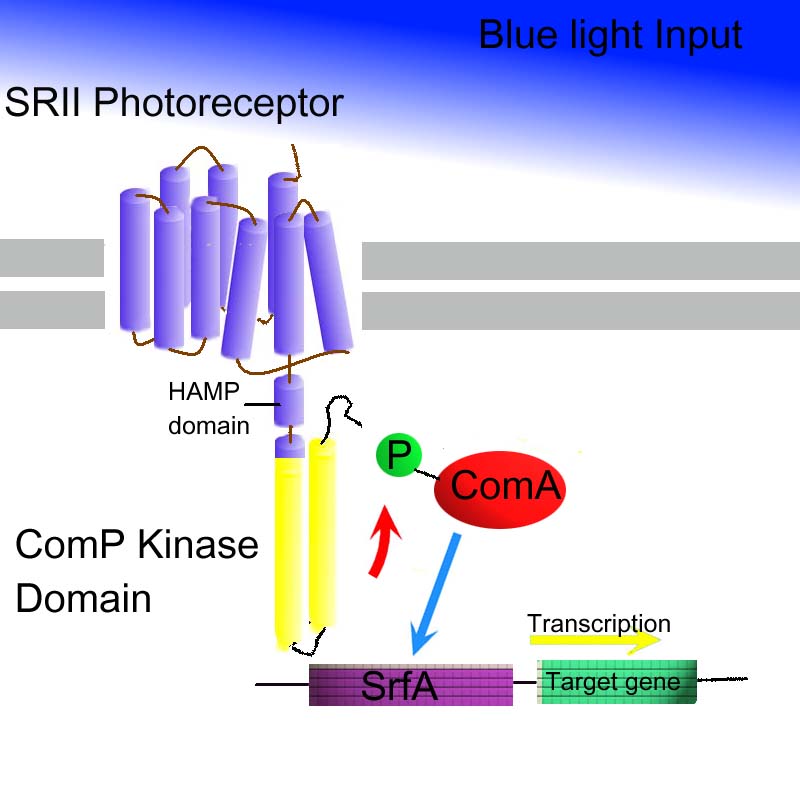
The kinase domain of HtrII will be replaced with the kinase domain of ComP. ComP forms part of a two-component system from Bacillus subtilis - and this will not affect any endogenous networks in e.coli. The two component system involves: ComP, a two-component sensor histidine kinase and ComA, a two-component response regulator. Phosphorylated comA will upregulate transcription at the psfA/srfA promoter psfA/srfA, as part of the AND gate.
In summary:
Contents |
Blue Photosensor Background
Method
Possible extensions:
- Determination of optimal wavelength:
- use of different substrates (different retinals)
- Separate variants all submitted as BioBricks.
- Submitted synthesized ComP and ComA as BioBricks
- Model the pathway to determine rate-limiting step
References
This part is based on “Photostimulation of a Sensory Rhodopsin II/HtrII/Tsr Fusion Chimera Activates CheA-Autophosphorylation and CheY-Phosphotransfer in Vitro” by Vishwa D. Trivedi and John L. Spudich, Biochemistry 2003, 42, 13887-13892. Acording to this article the peak sensitivity is to 500+/-5nm, and results in a 3 fold activation of the Tsr (wild type). CheA,W,Y connected system.
It is proposed to replace Tsr fusion with homolgouse ComP. SRII-HtrII fusion to which ComP is fused ComA when phosphorylated by ComP is an activator for PsfA promoter sequence from Dr Alan Grossman (M.I.T.) Based on
- SRII-HtrII-Tsr fusion from Prof J.L. Spudich (university of Texas)melb:spudich N sequence
- BBa_J51000 (ComP) kinase
- BBa_J51001 (ComA) activator
PARTS:
- SrfA promoter
- ComA protein generator
- SRII-ComP photosensor
- Any phyco construction genes?
SRII is from Natronomonas pharaonis.
Tsr fusion was made by Jung et al J Bacteriol 183 6365-6371 (2001) they propose a mechanism.