ETHZ/Simulations
From 2007.igem.org
m (→Model Parameters: aTc,TetR) |
(→Model Parameters) |
||
| (47 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
==== Constitutively produced proteins ==== | ==== Constitutively produced proteins ==== | ||
| - | [[Image: | + | [[Image:Model01b.png]] |
==== Learning system ==== | ==== Learning system ==== | ||
| - | [[Image: | + | [[Image:Model02b.png]] |
==== Reporter system ==== | ==== Reporter system ==== | ||
| - | [[Image: | + | [[Image:Model03b.png]] |
== System Equations == | == System Equations == | ||
| Line 17: | Line 17: | ||
==== Constitutively produced proteins ==== | ==== Constitutively produced proteins ==== | ||
| - | [[Image: | + | [[Image:Constitutive_braced.png|330px]] |
==== Learning system ==== | ==== Learning system ==== | ||
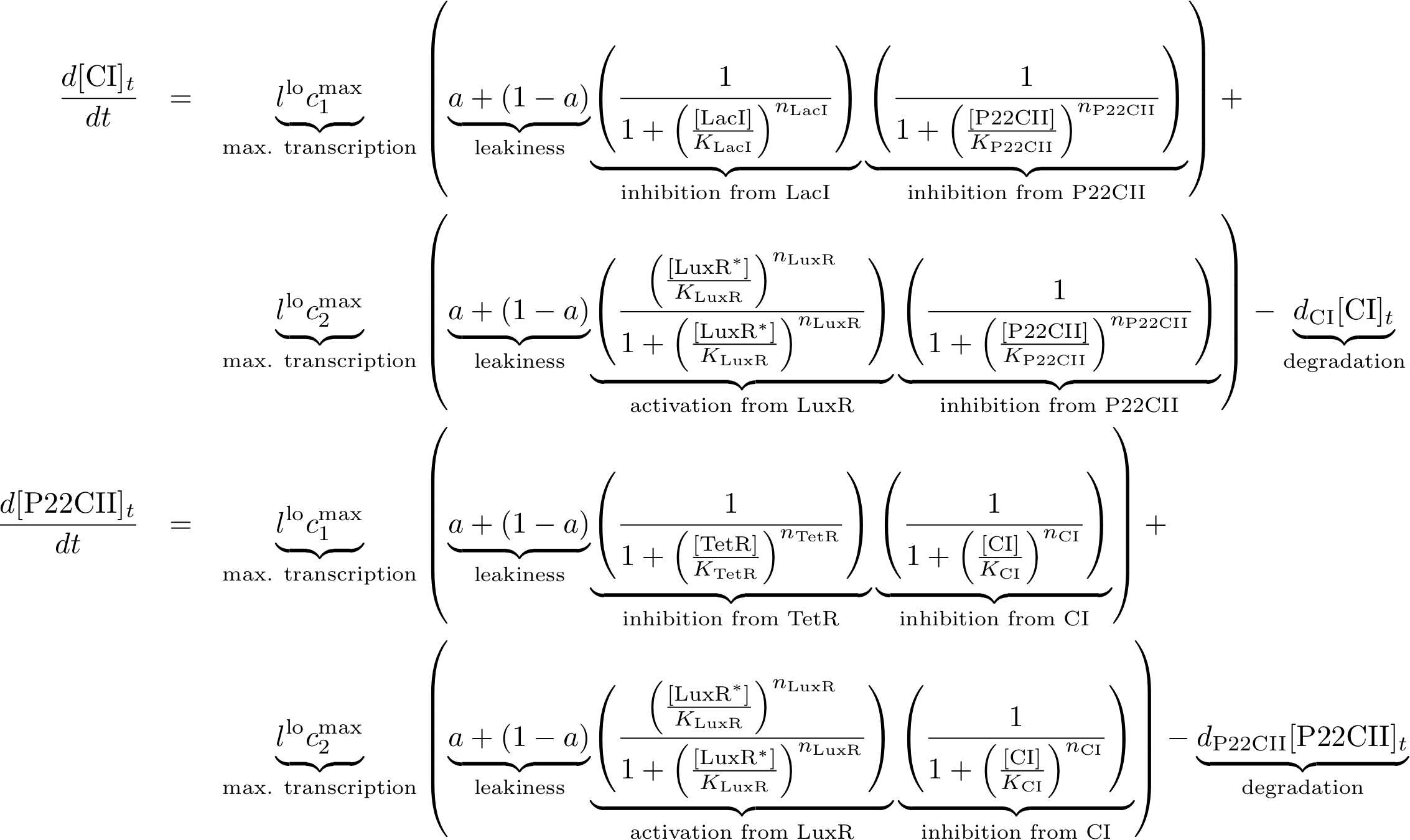
| - | [[Image: | + | [[Image:Toggle_braced.png|770px]] |
==== Reporter system ==== | ==== Reporter system ==== | ||
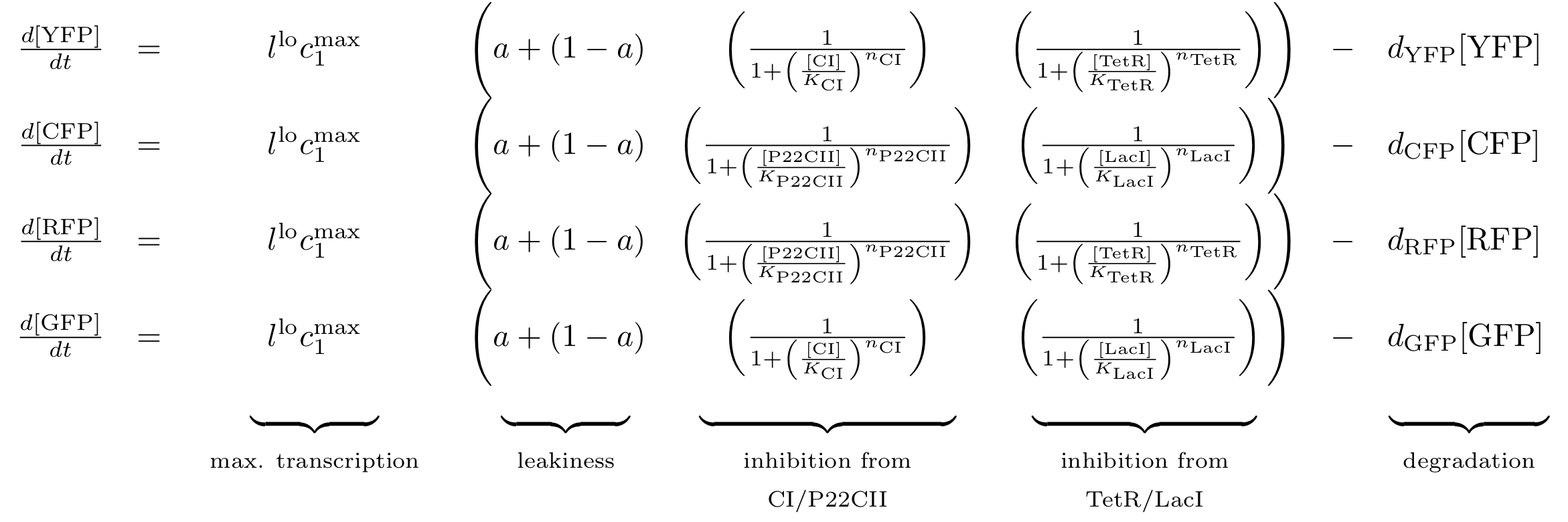
| - | [[Image: | + | [[Image:Reporter_braced.png|778px]] |
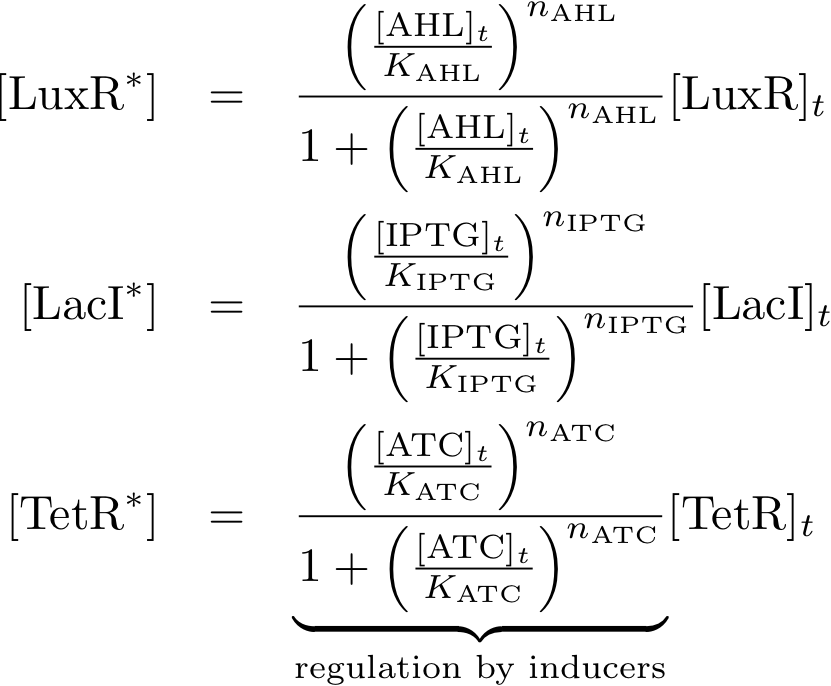
==== Allosteric regulation ==== | ==== Allosteric regulation ==== | ||
| Line 34: | Line 34: | ||
==== Comments ==== | ==== Comments ==== | ||
| - | Note that the three constitutively produced proteins | + | Note that the three constitutively produced proteins lacI, tetR and luxR exist in two different forms: as free proteins and in complexes they build with IPTG, aTc and AHL, respectively. |
In this new formulation of the model equations, the characterization is more amenable to human interpretation (although equivalent to the previous formuation). The promoters are now characterized by their ''maximum transcription rate'' (c<sub>i</sub><sup>max</sup>) and the ''basic production'' (a<sub>X</sub>), which gives the 'leakage' if the gene is fully inhibited. Note that in the given mathematical formulation the ''basic production'' is specified as a percentage of the ''max. transcription rate'' and is therefore unitless. | In this new formulation of the model equations, the characterization is more amenable to human interpretation (although equivalent to the previous formuation). The promoters are now characterized by their ''maximum transcription rate'' (c<sub>i</sub><sup>max</sup>) and the ''basic production'' (a<sub>X</sub>), which gives the 'leakage' if the gene is fully inhibited. Note that in the given mathematical formulation the ''basic production'' is specified as a percentage of the ''max. transcription rate'' and is therefore unitless. | ||
| Line 49: | Line 49: | ||
|- | |- | ||
| c<sub>1</sub><sup>max</sup> | | c<sub>1</sub><sup>max</sup> | ||
| - | | | + | | 0.01 [mM/h] |
| max. transcription rate of constitutive promoter (per gene) | | max. transcription rate of constitutive promoter (per gene) | ||
| - | | promoter no. J23105 | + | | promoter no. J23105; Reference: Estimate |
|- | |- | ||
| c<sub>2</sub><sup>max</sup> | | c<sub>2</sub><sup>max</sup> | ||
| - | | | + | | 0.01 [mM/h] |
| max. transcription rate of luxR-activated promoter (per gene) | | max. transcription rate of luxR-activated promoter (per gene) | ||
| - | | | + | | Reference: Estimate |
|- | |- | ||
| l<sup>hi</sup> | | l<sup>hi</sup> | ||
| 25 | | 25 | ||
| high-copy plasmid number | | high-copy plasmid number | ||
| - | | Reference: | + | | Reference: Estimate |
|- | |- | ||
| l<sup>lo</sup> | | l<sup>lo</sup> | ||
| 5 | | 5 | ||
| low-copy plasmid number | | low-copy plasmid number | ||
| - | | Reference: | + | | Reference: Estimate |
|- | |- | ||
| a<sub>Q<sub>2</sub>,R</sub> | | a<sub>Q<sub>2</sub>,R</sub> | ||
| 0.1 - 0.2 | | 0.1 - 0.2 | ||
| basic production of Q<sub>2</sub>/R-inhibited genes | | basic production of Q<sub>2</sub>/R-inhibited genes | ||
| - | | Reference: discussion | + | | Reference: Conclusions after discussion |
|- | |- | ||
| a<sub>Q<sub>2</sub></sub> | | a<sub>Q<sub>2</sub></sub> | ||
| 0.1 - 0.2 | | 0.1 - 0.2 | ||
| basic production of Q<sub>2</sub>-inhibited genes | | basic production of Q<sub>2</sub>-inhibited genes | ||
| - | | Reference: discussion | + | | Reference: Conclusions after discussion |
|- | |- | ||
| a<sub>Q<sub>1</sub>,S</sub> | | a<sub>Q<sub>1</sub>,S</sub> | ||
| 0.1 - 0.2 | | 0.1 - 0.2 | ||
| basic production of Q<sub>1</sub>/S-inhibited genes | | basic production of Q<sub>1</sub>/S-inhibited genes | ||
| - | | Reference: discussion | + | | Reference: Conclusions after discussion |
|- | |- | ||
| a<sub>Q<sub>1</sub></sub> | | a<sub>Q<sub>1</sub></sub> | ||
| 0.1 - 0.2 | | 0.1 - 0.2 | ||
| basic production of Q<sub>1</sub>-inhibited genes | | basic production of Q<sub>1</sub>-inhibited genes | ||
| - | | Reference: discussion | + | | Reference: Conclusions after discussion |
|- | |- | ||
| a<sub>Q<sub>2</sub>,S</sub> | | a<sub>Q<sub>2</sub>,S</sub> | ||
| 0.1 - 0.2 | | 0.1 - 0.2 | ||
| basic production of Q<sub>2</sub>/S-inhibited genes | | basic production of Q<sub>2</sub>/S-inhibited genes | ||
| - | | Reference: discussion | + | | Reference: Conclusions after discussion |
|- | |- | ||
| a<sub>Q<sub>1</sub>,R</sub> | | a<sub>Q<sub>1</sub>,R</sub> | ||
| 0.1 - 0.2 | | 0.1 - 0.2 | ||
| basic production of Q<sub>1</sub>/R-inhibited genes | | basic production of Q<sub>1</sub>/R-inhibited genes | ||
| - | | Reference: discussion | + | | Reference: Conclusions after discussion |
|- | |- | ||
| d<sub>R</sub> | | d<sub>R</sub> | ||
| - | | 2.31e-3 [ | + | | 2.31e-3 [per sec] |
| degradation of lacI | | degradation of lacI | ||
| - | | | + | | Ref. [10] |
|- | |- | ||
| d<sub>S</sub> | | d<sub>S</sub> | ||
| - | | 1e-5 [ | + | | |
| + | * 1e-5 [per sec] | ||
| + | * 2.31e-3 [per sec] | ||
| degradation of tetR | | degradation of tetR | ||
| - | | Ref | + | | |
| + | * Ref. [9] | ||
| + | * Ref. [10] | ||
|- | |- | ||
| d<sub>L</sub> | | d<sub>L</sub> | ||
| - | | | + | | 1e-3 - 1e-4 [per sec] |
| degradation of luxR | | degradation of luxR | ||
| + | | Ref: [6] | ||
|- | |- | ||
| d<sub>Q<sub>1</sub></sub> | | d<sub>Q<sub>1</sub></sub> | ||
| - | | 7e-4 [ | + | | 7e-4 [per sec] |
| degradation of cI | | degradation of cI | ||
| - | | Ref | + | | Ref. [7] |
|- | |- | ||
| d<sub>Q<sub>2</sub></sub> | | d<sub>Q<sub>2</sub></sub> | ||
| Line 124: | Line 129: | ||
| 6.3e-3 [per min] | | 6.3e-3 [per min] | ||
| degradation of YFP | | degradation of YFP | ||
| - | | suppl. mat. to | + | | suppl. mat. to Ref. [8] corresponding to a half life of 110min |
|- | |- | ||
| d<sub>GFP</sub> | | d<sub>GFP</sub> | ||
| Line 142: | Line 147: | ||
|- | |- | ||
| K<sub>R</sub> | | K<sub>R</sub> | ||
| - | | | + | | |
| + | * 0.1 - 1 [pM] | ||
| + | * 800 [nM] | ||
| lacI repressor dissociation constant | | lacI repressor dissociation constant | ||
| - | | | + | | |
| + | * Ref. [2] | ||
| + | * Ref. [12] | ||
|- | |- | ||
| K<sub>I<sub>R</sub></sub> | | K<sub>I<sub>R</sub></sub> | ||
| - | | 1. | + | | 1.3 [µM] |
| IPTG-lacI repressor dissociation constant | | IPTG-lacI repressor dissociation constant | ||
| - | | Ref. [ | + | | Ref. [2] |
|- | |- | ||
| K<sub>S</sub> | | K<sub>S</sub> | ||
| - | | | + | | 179 [pM] |
| tetR repressor dissociation constant | | tetR repressor dissociation constant | ||
| - | | Ref. [1] | + | | Ref. [1] |
|- | |- | ||
| K<sub>I<sub>S</sub></sub> | | K<sub>I<sub>S</sub></sub> | ||
| - | | | + | | 893 [pM] |
| aTc-tetR repressor dissociation constant | | aTc-tetR repressor dissociation constant | ||
| - | | Ref. [1] | + | | Ref. [1] |
|- | |- | ||
| K<sub>L</sub> | | K<sub>L</sub> | ||
| - | | | + | | |
| + | * 55 - 520 [nM] | ||
| + | * 10 [nM] | ||
| luxR activator dissociation constant | | luxR activator dissociation constant | ||
| + | | | ||
| + | * Ref: [6] | ||
| + | * Ref: [12] | ||
|- | |- | ||
| K<sub>I<sub>L</sub></sub> | | K<sub>I<sub>L</sub></sub> | ||
| - | | | + | | 0.09 - 1 [µM] |
| AHL-luxR activator dissociation constant | | AHL-luxR activator dissociation constant | ||
| + | | Ref: [6] | ||
|- | |- | ||
| K<sub>Q<sub>1</sub></sub> | | K<sub>Q<sub>1</sub></sub> | ||
| - | | | + | | |
| + | * 8 [pM] | ||
| + | * 50 [nM] | ||
| cI repressor dissociation constant | | cI repressor dissociation constant | ||
| - | | Ref. [ | + | | |
| + | * Ref. [12] | ||
| + | * starting with values of Ref. [6] and using Ref. [3] | ||
|- | |- | ||
| K<sub>Q<sub>2</sub></sub> | | K<sub>Q<sub>2</sub></sub> | ||
| - | | | + | | 0.577 [µM] |
| p22cII repressor dissociation constant | | p22cII repressor dissociation constant | ||
| + | | Ref. [11]. Note that they use a protein cII and we have p22cII. Does that match? It matches. p22 cII just means its cII derived frome a p22 vector ;-) | ||
|- | |- | ||
| n<sub>R</sub> | | n<sub>R</sub> | ||
| - | | 1 | + | | |
| + | * 1 | ||
| + | * 2 | ||
| lacI repressor Hill cooperativity | | lacI repressor Hill cooperativity | ||
| - | | Ref. [5] | + | | |
| + | * Ref. [5] | ||
| + | * Ref. [12] | ||
|- | |- | ||
| n<sub>I<sub>R</sub></sub> | | n<sub>I<sub>R</sub></sub> | ||
| Line 199: | Line 223: | ||
|- | |- | ||
| n<sub>L</sub> | | n<sub>L</sub> | ||
| - | | | + | | 2 |
| luxR activator Hill cooperativity | | luxR activator Hill cooperativity | ||
| - | | Ref | + | | Ref: [6] |
|- | |- | ||
| n<sub>I<sub>L</sub></sub> | | n<sub>I<sub>L</sub></sub> | ||
| Line 209: | Line 233: | ||
|- | |- | ||
| n<sub>Q<sub>1</sub></sub> | | n<sub>Q<sub>1</sub></sub> | ||
| - | | | + | | 2 |
| cI repressor Hill cooperativity | | cI repressor Hill cooperativity | ||
| - | | Ref. [ | + | | Ref. [12] |
|- | |- | ||
| n<sub>Q<sub>2</sub></sub> | | n<sub>Q<sub>2</sub></sub> | ||
| - | | | + | | 4 |
| p22cII repressor Hill cooperativity | | p22cII repressor Hill cooperativity | ||
| + | | Ref. [11]. Note that they use a protein cII and we have p22cII. Does that match? It matches. p22 cII just means its cII derived frome a p22 vector ;-) | ||
|- | |- | ||
|} | |} | ||
== References == | == References == | ||
| - | + | [http://www.pnas.org/cgi/content/abstract/104/8/2643 [1] Weber W et al.] <i>"A synthetic time-delay circuit in mammalian cells and mice"</i>, P Natl Acad Sci USA 104(8):2643-2648, 2007<br /> | |
| - | + | [http://www.pnas.org/cgi/content/full/100/13/7702?ck=nck [2] Setty Y et al.] <i>"Detailed map of a cis-regulatory input function"</i>, P Natl Acad Sci USA 100(13):7702-7707, 2003<br /> | |
| - | # | + | [http://ieeexplore.ieee.org/iel5/9711/30654/01416417.pdf [3] Braun D et al.] <i>"Parameter Estimation for Two Synthetic Gene Networks: A Case Study"</i>, ICASSP 5:769-772, 2005<br /> |
| - | # | + | [http://www.nature.com/nature/journal/v435/n7038/suppinfo/nature03508.html [4] Fung E et al.] <i>"A synthetic gene--metabolic oscillator"</i>, Nature 435:118-122, 2005 (supplementary material)<br /> |
| - | # | + | [http://dx.doi.org/10.1016/j.jbiotec.2005.08.030 [5] Iadevaia S and Mantzais NV] <i>"Genetic network driven control of PHBV copolymer composition"</i>, J Biotechnol 122(1):99-121, 2006<br /> |
| - | # Genetic network driven control of PHBV copolymer composition (http://doi | + | [http://dx.doi.org/10.1016/j.biosystems.2005.04.006 [6] Goryachev AB et al.] <i>"Systems analysis of a quorum sensing network: Design constraints imposed by the functional requirements, network topology and kinetic constants"</i>, Biosystems 83(2-3):178-187, 2004<br /> |
| + | [http://www.genetics.org/cgi/content/abstract/149/4/1633 [7] Arkin A et al.] <i>"Stochastic kinetic analysis of developmental pathway bifurcation in phage λ-Infected Escherichia coli cells"</i>, Genetics 149: 1633-1648, 1998<br /> | ||
| + | [http://download.cell.com/supplementarydata/cell/107/6/739/DC1/index.htm [8] Colman-Lerner A et al.] <i>"Yeast Cbk1 and Mob2 Activate Daughter-Specific Genetic Programs to Induce Asymmetric Cell Fates"</i>, Cell 107(6): 739-750, 2001 (supplementary material)<br /> | ||
| + | [http://www.nature.com/nature/journal/v405/n6786/abs/405590a0.html [9] Becskei A and Serrano L] <i>"Engineering stability in gene networks by autoregulation"</i>, Nature 405: 590-593, 2000<br /> | ||
| + | [http://www.biophysj.org/cgi/content/full/89/6/3873?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&searchid=1&FIRSTINDEX=0&volume=89&firstpage=3873&resourcetype=HWCIT [10] Tuttle et al.] <i>"Model-Driven Designs of an Oscillating Gene Network"</i>, Biophys J 89(6):3873-3883, 2005<br /> | ||
| + | [http://www.pnas.org/cgi/reprint/99/2/679 [11] McMillen LM et al.] <i>"Synchronizing genetic relaxation oscillators by intercell signaling"</i>, P Natl Acad Sci USA 99(2):679-684, 2002<br /> | ||
| + | [http://www.nature.com/nature/journal/v434/n7037/full/nature03461.html [12] Basu S et al.] <i>"A synthetic multicellular system for programmed pattern formation"</i>, Nature 434:1130-1134, 2005<br /> | ||
== Variable Mapping == | == Variable Mapping == | ||
Latest revision as of 12:55, 18 October 2007
Contents |
Basic Model
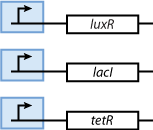
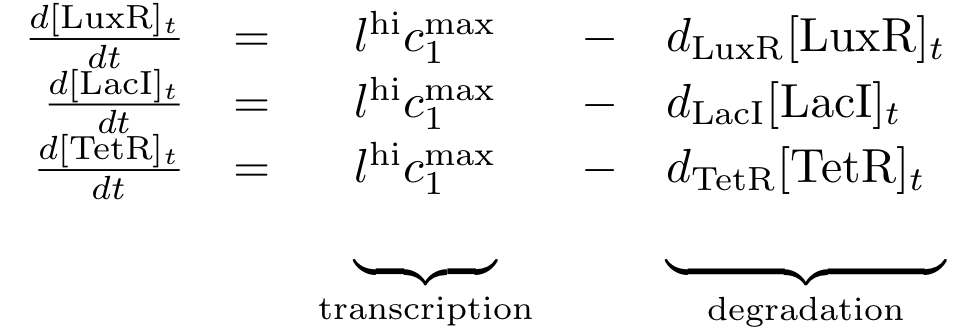
Constitutively produced proteins
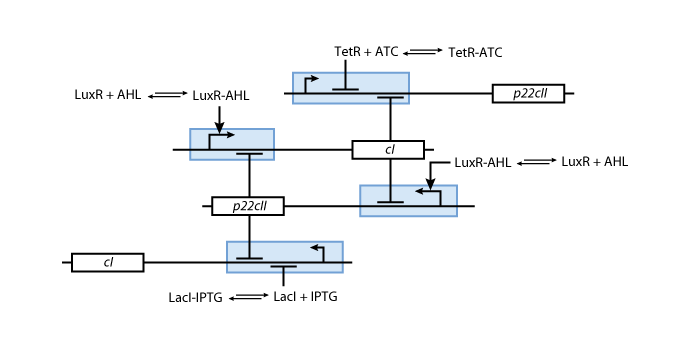
Learning system
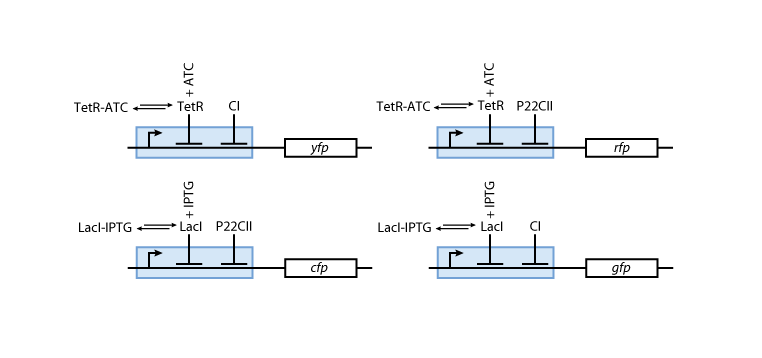
Reporter system
System Equations
Constitutively produced proteins
Learning system
Reporter system
Allosteric regulation
Comments
Note that the three constitutively produced proteins lacI, tetR and luxR exist in two different forms: as free proteins and in complexes they build with IPTG, aTc and AHL, respectively.
In this new formulation of the model equations, the characterization is more amenable to human interpretation (although equivalent to the previous formuation). The promoters are now characterized by their maximum transcription rate (cimax) and the basic production (aX), which gives the 'leakage' if the gene is fully inhibited. Note that in the given mathematical formulation the basic production is specified as a percentage of the max. transcription rate and is therefore unitless.
The max. transcription rate is given per gene (as agreed with Sven during the meeting at Sep 20.). This means that to get the total transcription rate we need to multiply with the number of gene copies per cell which is represented as llo/lhi in the model equations.
Model Parameters
| Parameter | Value | Description | Comments |
|---|---|---|---|
| c1max | 0.01 [mM/h] | max. transcription rate of constitutive promoter (per gene) | promoter no. J23105; Reference: Estimate |
| c2max | 0.01 [mM/h] | max. transcription rate of luxR-activated promoter (per gene) | Reference: Estimate |
| lhi | 25 | high-copy plasmid number | Reference: Estimate |
| llo | 5 | low-copy plasmid number | Reference: Estimate |
| aQ2,R | 0.1 - 0.2 | basic production of Q2/R-inhibited genes | Reference: Conclusions after discussion |
| aQ2 | 0.1 - 0.2 | basic production of Q2-inhibited genes | Reference: Conclusions after discussion |
| aQ1,S | 0.1 - 0.2 | basic production of Q1/S-inhibited genes | Reference: Conclusions after discussion |
| aQ1 | 0.1 - 0.2 | basic production of Q1-inhibited genes | Reference: Conclusions after discussion |
| aQ2,S | 0.1 - 0.2 | basic production of Q2/S-inhibited genes | Reference: Conclusions after discussion |
| aQ1,R | 0.1 - 0.2 | basic production of Q1/R-inhibited genes | Reference: Conclusions after discussion |
| dR | 2.31e-3 [per sec] | degradation of lacI | Ref. [10] |
| dS |
| degradation of tetR |
|
| dL | 1e-3 - 1e-4 [per sec] | degradation of luxR | Ref: [6] |
| dQ1 | 7e-4 [per sec] | degradation of cI | Ref. [7] |
| dQ2 | degradation of p22cII | ||
| dYFP | 6.3e-3 [per min] | degradation of YFP | suppl. mat. to Ref. [8] corresponding to a half life of 110min |
| dGFP | 6.3e-3 [per min] | degradation of GFP | in analogy to YFP |
| dRFP | 6.3e-3 [per min] | degradation of RFP | in analogy to YFP |
| dCFP | 6.3e-3 [per min] | degradation of CFP | in analogy to YFP |
| KR |
| lacI repressor dissociation constant |
|
| KIR | 1.3 [µM] | IPTG-lacI repressor dissociation constant | Ref. [2] |
| KS | 179 [pM] | tetR repressor dissociation constant | Ref. [1] |
| KIS | 893 [pM] | aTc-tetR repressor dissociation constant | Ref. [1] |
| KL |
| luxR activator dissociation constant |
|
| KIL | 0.09 - 1 [µM] | AHL-luxR activator dissociation constant | Ref: [6] |
| KQ1 |
| cI repressor dissociation constant |
|
| KQ2 | 0.577 [µM] | p22cII repressor dissociation constant | Ref. [11]. Note that they use a protein cII and we have p22cII. Does that match? It matches. p22 cII just means its cII derived frome a p22 vector ;-) |
| nR |
| lacI repressor Hill cooperativity |
|
| nIR | 2 | IPTG-lacI repressor Hill cooperativity | Ref. [5] |
| nS | 3 | tetR repressor Hill cooperativity | Ref. [3] |
| nIS | 2 (1.5-2.5) | aTc-tetR repressor Hill cooperativity | Ref. [3] |
| nL | 2 | luxR activator Hill cooperativity | Ref: [6] |
| nIL | 1 | AHL-luxR activator Hill cooperativity | Ref. [3] |
| nQ1 | 2 | cI repressor Hill cooperativity | Ref. [12] |
| nQ2 | 4 | p22cII repressor Hill cooperativity | Ref. [11]. Note that they use a protein cII and we have p22cII. Does that match? It matches. p22 cII just means its cII derived frome a p22 vector ;-) |
References
[http://www.pnas.org/cgi/content/abstract/104/8/2643 [1] Weber W et al.] "A synthetic time-delay circuit in mammalian cells and mice", P Natl Acad Sci USA 104(8):2643-2648, 2007
[http://www.pnas.org/cgi/content/full/100/13/7702?ck=nck [2] Setty Y et al.] "Detailed map of a cis-regulatory input function", P Natl Acad Sci USA 100(13):7702-7707, 2003
[http://ieeexplore.ieee.org/iel5/9711/30654/01416417.pdf [3] Braun D et al.] "Parameter Estimation for Two Synthetic Gene Networks: A Case Study", ICASSP 5:769-772, 2005
[http://www.nature.com/nature/journal/v435/n7038/suppinfo/nature03508.html [4] Fung E et al.] "A synthetic gene--metabolic oscillator", Nature 435:118-122, 2005 (supplementary material)
[http://dx.doi.org/10.1016/j.jbiotec.2005.08.030 [5] Iadevaia S and Mantzais NV] "Genetic network driven control of PHBV copolymer composition", J Biotechnol 122(1):99-121, 2006
[http://dx.doi.org/10.1016/j.biosystems.2005.04.006 [6] Goryachev AB et al.] "Systems analysis of a quorum sensing network: Design constraints imposed by the functional requirements, network topology and kinetic constants", Biosystems 83(2-3):178-187, 2004
[http://www.genetics.org/cgi/content/abstract/149/4/1633 [7] Arkin A et al.] "Stochastic kinetic analysis of developmental pathway bifurcation in phage λ-Infected Escherichia coli cells", Genetics 149: 1633-1648, 1998
[http://download.cell.com/supplementarydata/cell/107/6/739/DC1/index.htm [8] Colman-Lerner A et al.] "Yeast Cbk1 and Mob2 Activate Daughter-Specific Genetic Programs to Induce Asymmetric Cell Fates", Cell 107(6): 739-750, 2001 (supplementary material)
[http://www.nature.com/nature/journal/v405/n6786/abs/405590a0.html [9] Becskei A and Serrano L] "Engineering stability in gene networks by autoregulation", Nature 405: 590-593, 2000
[http://www.biophysj.org/cgi/content/full/89/6/3873?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&searchid=1&FIRSTINDEX=0&volume=89&firstpage=3873&resourcetype=HWCIT [10] Tuttle et al.] "Model-Driven Designs of an Oscillating Gene Network", Biophys J 89(6):3873-3883, 2005
[http://www.pnas.org/cgi/reprint/99/2/679 [11] McMillen LM et al.] "Synchronizing genetic relaxation oscillators by intercell signaling", P Natl Acad Sci USA 99(2):679-684, 2002
[http://www.nature.com/nature/journal/v434/n7037/full/nature03461.html [12] Basu S et al.] "A synthetic multicellular system for programmed pattern formation", Nature 434:1130-1134, 2005
Variable Mapping
| Variable | Compound |
|---|---|
| R | lacI |
| IR | IPTG |
| S | tetR |
| IS | aTc |
| L | luxR |
| IL | AHL |
| Q1 | cI |
| Q2 | p22cII |