NYMU Taipei/Lab Notes/2007 9 27
From 2007.igem.org
< NYMU Taipei/Lab Notes(Difference between revisions)
(→pOmpC glucose response testing by construct (pOmpC + EYFP)) |
(→CinR+HSL+D-term check) |
||
| (6 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
* sample at each lane | * sample at each lane | ||
** 1Kb ladder | ** 1Kb ladder | ||
| - | ** block #1 (insert band appears) | + | ** block #1 (insert band appears), 0.41 ug/uL |
** block #2 (insert band appears, and one extra band around 400 bp) | ** block #2 (insert band appears, and one extra band around 400 bp) | ||
| - | ** block #3 (insert band appears) | + | ** block #3 (insert band appears), 0.25 ug/uL |
| - | ** block #4 (insert band appears) | + | ** block #4 (insert band appears), 0.41 ug/uL |
** block #5 (insert band does not appear, only band around 400 bp) | ** block #5 (insert band does not appear, only band around 400 bp) | ||
** block #6 (insert band does not appear, only band around 400 bp) | ** block #6 (insert band does not appear, only band around 400 bp) | ||
| - | * | + | * expected results: one band at (1,623 + 316) bp is our insert |
** insert = RBS+CinR (2,843 - 2,053) + RBS+HSL (2,783 - 2,053) + D-term (3,266 - 3,163) | ** insert = RBS+CinR (2,843 - 2,053) + RBS+HSL (2,783 - 2,053) + D-term (3,266 - 3,163) | ||
** insert = 790 + 730 + 103 = 1,623 | ** insert = 790 + 730 + 103 = 1,623 | ||
| Line 20: | Line 20: | ||
[[Image:NYMU Taipei 20070927 CinR-HSL-D-term plate culture test.jpg]] | [[Image:NYMU Taipei 20070927 CinR-HSL-D-term plate culture test.jpg]] | ||
---- | ---- | ||
| + | |||
==pOmpC glucose response testing by construct (pOmpC + EYFP)== | ==pOmpC glucose response testing by construct (pOmpC + EYFP)== | ||
| - | * design: 5 tubes for 0%, 1%, 3%, 5%, 10% extra glucose cultured in LB medium | + | * design: 5 tubes for 0%, 1%, 3%, 5%, 10% extra glucose cultured in LB medium (total volume 5 mL = 5,000 uL) |
* tube with 0% extra glucose is treated as negative control | * tube with 0% extra glucose is treated as negative control | ||
| - | {| align=center | border=1 | + | * 20% glucose solution |
| + | {| align=center | border=1 | style="text-align:center" | ||
!glucose (%) | !glucose (%) | ||
!O.D. 600 | !O.D. 600 | ||
!white light | !white light | ||
!green light | !green light | ||
| + | !LB volume (uL) | ||
| + | !glucose volume (uL) | ||
|- | |- | ||
|0 | |0 | ||
| Line 33: | Line 37: | ||
|[[Image:NYMU Taipei pOmpC-EYFP 0p-w.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 0p-w.JPG|100px]] | ||
|[[Image:NYMU Taipei pOmpC-EYFP 0p-g2.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 0p-g2.JPG|100px]] | ||
| + | |5,000 | ||
| + | |0 | ||
|- | |- | ||
|1 | |1 | ||
| Line 38: | Line 44: | ||
|[[Image:NYMU Taipei pOmpC-EYFP 1p-w.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 1p-w.JPG|100px]] | ||
|[[Image:NYMU Taipei pOmpC-EYFP 1p-g3.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 1p-g3.JPG|100px]] | ||
| + | |4,750 | ||
| + | |250 | ||
|- | |- | ||
|3 | |3 | ||
| Line 43: | Line 51: | ||
|[[Image:NYMU Taipei pOmpC-EYFP 3p-w.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 3p-w.JPG|100px]] | ||
|[[Image:NYMU Taipei pOmpC-EYFP 3p-g.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 3p-g.JPG|100px]] | ||
| + | |4,250 | ||
| + | |750 | ||
|- | |- | ||
|5 | |5 | ||
| Line 48: | Line 58: | ||
|[[Image:NYMU Taipei pOmpC-EYFP 5p-w.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 5p-w.JPG|100px]] | ||
|[[Image:NYMU Taipei pOmpC-EYFP 5p-g.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 5p-g.JPG|100px]] | ||
| + | |3,750 | ||
| + | |1,250 | ||
|- | |- | ||
|10 | |10 | ||
| Line 53: | Line 65: | ||
|[[Image:NYMU Taipei pOmpC-EYFP 10p-w.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 10p-w.JPG|100px]] | ||
|[[Image:NYMU Taipei pOmpC-EYFP 10p-g.JPG|100px]] | |[[Image:NYMU Taipei pOmpC-EYFP 10p-g.JPG|100px]] | ||
| + | |2,500 | ||
| + | |2,500 | ||
|- | |- | ||
|} | |} | ||
Latest revision as of 12:02, 3 October 2007
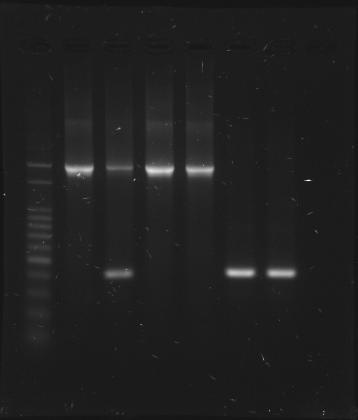
CinR+HSL+D-term check
- After re-culture in 20-block plate,randomly pick 6 blocks from CinR+HSL+D-term plate
- PCR check using VF2, VR primers
- PCR total 50 uL, 10 uL for GEL separation
- sample at each lane
- 1Kb ladder
- block #1 (insert band appears), 0.41 ug/uL
- block #2 (insert band appears, and one extra band around 400 bp)
- block #3 (insert band appears), 0.25 ug/uL
- block #4 (insert band appears), 0.41 ug/uL
- block #5 (insert band does not appear, only band around 400 bp)
- block #6 (insert band does not appear, only band around 400 bp)
- expected results: one band at (1,623 + 316) bp is our insert
- insert = RBS+CinR (2,843 - 2,053) + RBS+HSL (2,783 - 2,053) + D-term (3,266 - 3,163)
- insert = 790 + 730 + 103 = 1,623
- extra band at 400 bp might be plasmid failed to be correctly ligated
- PCR product length without any insert = (3,189 - 3,049) + 176 = 316
- References
- [http://partsregistry.org/Image:PsB1AK3_map.jpg vector map of pSB1AK3 and VF2 VR primers]
pOmpC glucose response testing by construct (pOmpC + EYFP)
- design: 5 tubes for 0%, 1%, 3%, 5%, 10% extra glucose cultured in LB medium (total volume 5 mL = 5,000 uL)
- tube with 0% extra glucose is treated as negative control
- 20% glucose solution
| glucose (%) | O.D. 600 | white light | green light | LB volume (uL) | glucose volume (uL) |
|---|---|---|---|---|---|
| 0 | 2.487 | 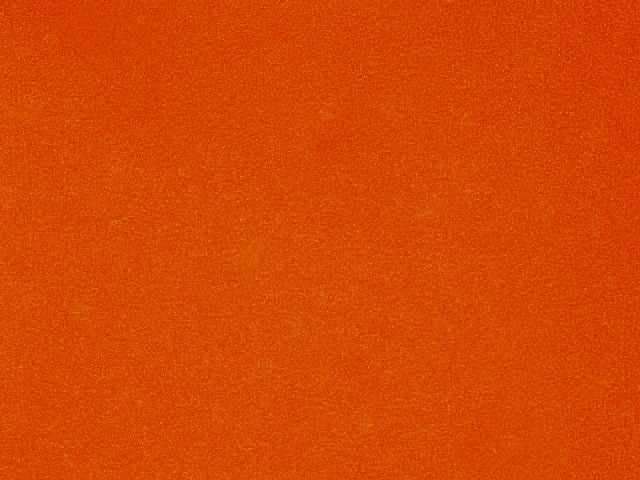
| 
| 5,000 | 0 |
| 1 | 2.091 | 
| 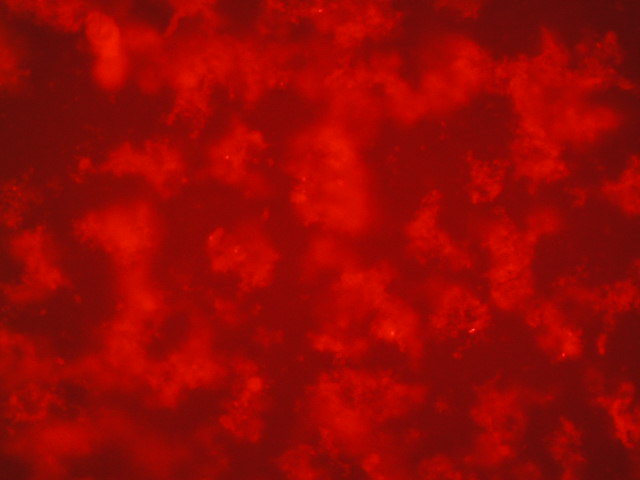
| 4,750 | 250 |
| 3 | 1.861 | 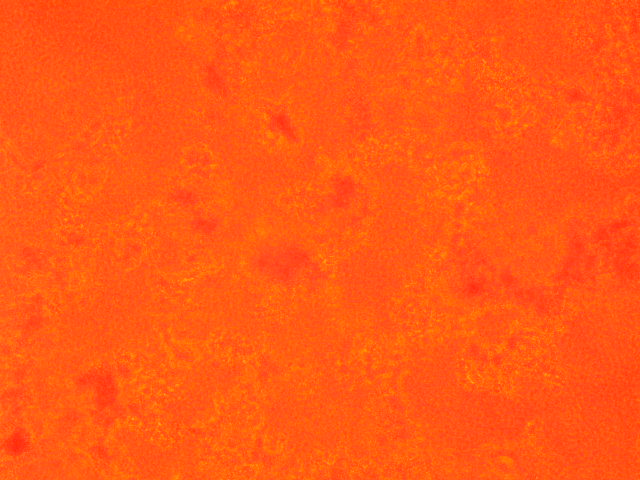
| 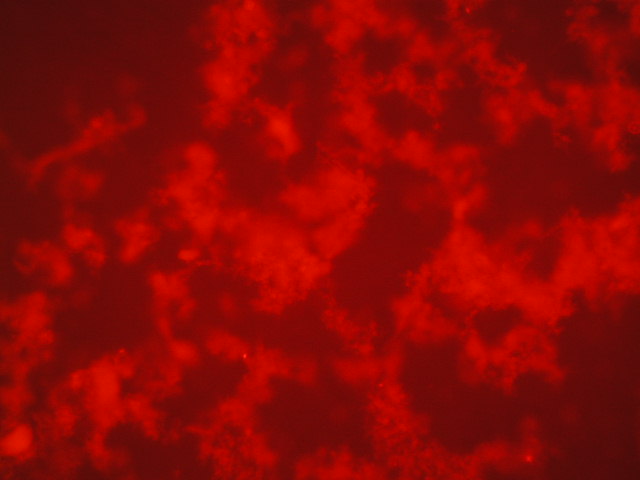
| 4,250 | 750 |
| 5 | 1.637 | 
| 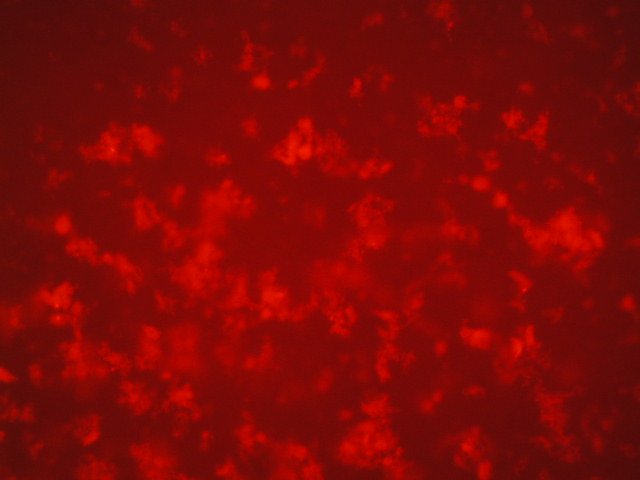
| 3,750 | 1,250 |
| 10 | 1.025 | 
| 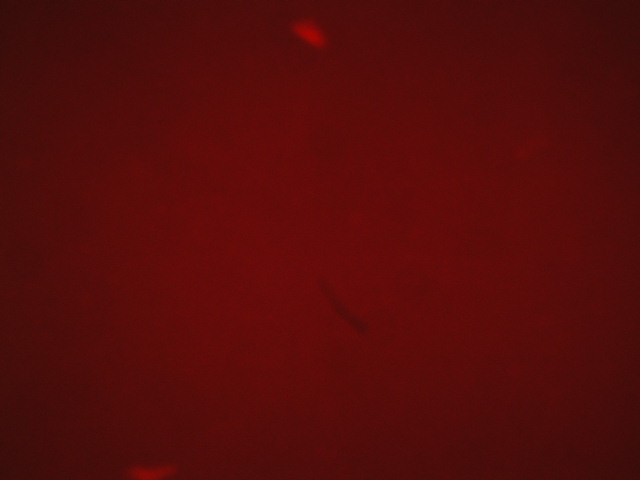
| 2,500 | 2,500 |