Arthur Yu Notebook
From 2007.igem.org
JCAnderson (Talk | contribs) |
|||
| (76 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
[[Template:BerkiGEM2007_ArthurConstructionFiles | My Construction Files]]<br> | [[Template:BerkiGEM2007_ArthurConstructionFiles | My Construction Files]]<br> | ||
| - | [[Template:BerkiGEM2007_ArthurSequencingFiles | My Sequencing Files]]<br> | + | [[Template:BerkiGEM2007_ArthurSequencingFiles | My Sequencing Files]]<br><br> |
---- | ---- | ||
| - | == | + | ==8/15 so Zinc represses growth== |
| - | + | * it represses growth! oh nooooo | |
| + | * will leave assay things going overnight | ||
| + | * retrasnsformed I716034 because i am suspicious of the -80 pir116 i made earlier | ||
| + | ==8/14 hurrah== | ||
| + | * I716034 "knockin'" is xformed into pir116 and plated. hopefully it works. | ||
| + | * so this pzhuC thing works with 20x the zinc used before (i think you need 20 uL of 1M ZnCl2 per each 1 mL) | ||
| + | * growth curve assay says... the things with zinc stopped growth at 0.06 OD. what????? | ||
| + | * incubating various 5 mL tubies of varying zinc added. | ||
| + | ==8/13 status report== | ||
| + | * pzhuC: 8 different clones stuck with zinc to see if any turn off zincyness | ||
| + | # also 30.la and 30.2b put into varying concentrations Zn2+ | ||
| + | * wbbl/neus into genome: incubated some clones and will xform tmrw. made competant pir116s today (too lightly colored solution?) | ||
| + | ''todo'': catelogue my boxes. make presentation draft draft. make t-shirts/other clothing | ||
| + | ==8/7 good productivity== | ||
| + | * so what have I done recently? | ||
| + | # I716026 pznuC (Zn2+ repressed promoter) and I716030 composite with RFP. The RFP is incubating right now | ||
| + | # I716028 pmgrB (Mg2+ repressed promoter) and I716031 w/RFP this is where I try to cut off the rbs to just get a basic promoter part. | ||
| + | # I716028 pmgrB and I716032 also incubating. This is where I try to inclube the RBS. Stops before the ORF in the MG1655 genome. | ||
| + | # wbbL neuS - assay worked great, so my clone of I716023 is good (however it's Bbriock v1.0). Currrently working on integrating it into the genome. Just transformed into DH10B today, and plated. | ||
| + | ''Other things:'' two bottles of LB went bad (???). somebody jacked my red pipetter. we need more lb/amp plates ._. | ||
| + | ==8/3 dry ice dry ice dry ice dry ice== | ||
| + | * 27 28 29 plated | ||
| + | * wbbL/neuS: incubating. ON culture will be used tmrw in assay. | ||
| + | * plates | ||
| + | ==8/1 AUG 1 '07 telebears pt 2== | ||
| + | * I716023 miniprepped and transformed into MC828 for O antigen testing | ||
| + | * I716027 picked 4 guys and incubating | ||
| + | * PCR did before leaving, for znuC and mgrB promoters. | ||
| + | ==7/31 my green pipette is missing== | ||
| + | * wbbL/neuS assay on I716023 (w/ and w/o K1 phage) | ||
| + | # one clone worked! it's the lysed one in the following picture | ||
| + | [[Image:073107_assay.jpg]] | ||
| + | * made oligos for | ||
| + | # Vitreosilla sp. VHb hemoglobin | ||
| + | # zinc promoter | ||
| + | # Mg promoter | ||
| + | |||
| + | ==7/30 vitreoscilla rly?== | ||
| + | * Made I716023, which is the wbbL neuS biobrick 1.0 style. | ||
| + | * did some reading to find promoter for sam | ||
| + | ==7/26 still trying== | ||
| + | * I716020 repeat creation is funny, not white. colony pcr says... | ||
| + | [[Image:072607_gel1.jpg|300px]] | ||
| + | * I716008 sent for reverse sequencing to get a complete picture (is it really good? am I just messing up in making I716012?) | ||
| + | |||
| + | ==7/25 few have won== | ||
| + | * I716020 not successfully made. none were red, and the E/Ba digest gel looked really really funny (4.7k, 3k, 2k; expected was 4.9k, 0.9k) | ||
| + | * it was religated and we will see tomorrow how the plate is | ||
| + | * I716012 lol k put in incushaker tubes, we will see tomorrow how the tubes is | ||
| + | |||
| + | ==7/24 many will enter few will win== | ||
| + | * There was one clone of the I716008 that looked good: R4 | ||
| + | * a test digest suggests that it is 100% correct | ||
| + | * But the triple digest, while having a 2150 band that I wanted, seemed to only have one 1100 or 900 band. Weird | ||
| + | * transformed into MC828 and MC828E anyway, hopefully it works tmrw | ||
| + | ==7/23 another day== | ||
| + | * iron "UCB" "IGEM 07" redone | ||
| + | * I716008 9 minipreps done, each a diff clone, and sent for forward sequencings. | ||
| + | * I716020 plated. | ||
| + | ==7/20 Untitled 2== | ||
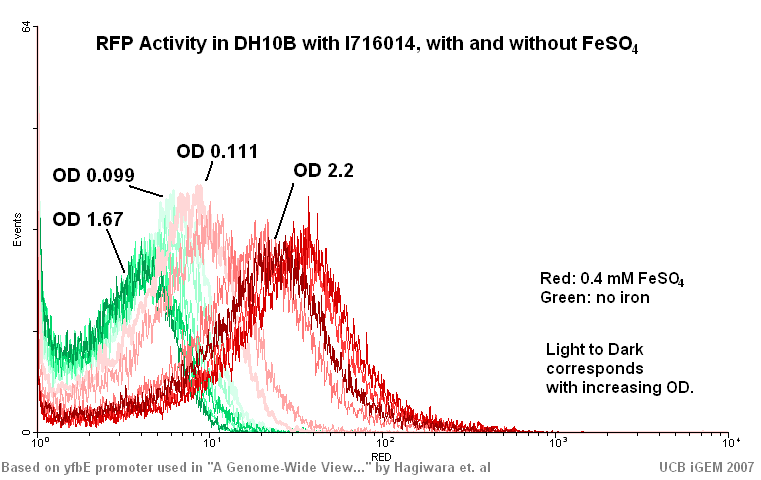
| + | * I716019 found to be created correctly. (t7-rbs-cytB5-rbs-cytB5red-dblterm) see ay42 and ay43 | ||
| + | [[Image:072007_yfbEgraph.jpg]] | ||
| + | <br> | ||
| + | '''[[072007_neg | White Cell Negative]]''' | ||
| + | |||
| + | ==7/19 Untitled== | ||
| + | * so the sequencing for wbbL/neuS showed up really mixy and funny. Seems like 1/8 to 1/4 of the library has success though, so I will replate and then mini individual colonies and sequence each one til I find a winner. | ||
| + | * yfbE assay was a success. Crisis averted by finding "Laser On" option in FlowCyto program. | ||
| + | * cytB5/red cassette completed and sent to quintara for sequencing. | ||
| + | ==7/18 A's take Rangers to school== | ||
| + | * A's mediocre, Rangers pretty bad | ||
| + | * I put 12 into the wrong cell >_< well sequencing shows that I'm missing half the promoter anyway so... meh | ||
| + | * yfbE iron promoter workses (P series). See pic on right. [[Image:071807_yfbE.jpg|300px|right]] | ||
| + | ==7/17 yaeyeayea == | ||
| + | * iron stuff growing tubes. 16 tubes, 4+4 clones jp style and not | ||
| + | * 12 and 19 are growing plate. | ||
| + | ==7/16 four ligations== | ||
| + | * the sequencing for wbbL/neuS library seems like Ptet is cut out. bleh doing again | ||
| + | * made I716008, I716018, I716017, I716016 and plated | ||
| + | ==7/13 friday== | ||
| + | * the RFP without ATG looks good (sequencing) | ||
| + | * wbbl/neuS lib didn't work again hmm, sent for sequencing | ||
| + | * other stuff will insert later | ||
| + | ==7/12 efficiency== | ||
| + | [[Image:071207_ayu_gel2.jpg|100px|right]] | ||
| + | *got news that I716012 didn't sequence well, trying amplify | ||
| + | *incubated I716015 (pBca9145-RFP_noATG) | ||
| + | *miniprepped yfbE's (I716013 and I716014) | ||
| + | *plated proprietary I716016 on FeCl3 and without | ||
| + | *new yfbE stuff for sequencing | ||
| + | *running gel on I716015 pcr prod to expediate testing | ||
| + | *redo I716012 again | ||
| + | # digesting 101 and 008 | ||
| + | # growing up electrocompetant MC828E | ||
| + | |||
| + | remarks | ||
| + | austin said cytochrome b5 /b5 red was pretty. yeayeayeayaeyea | ||
| + | ==7/11 seven eleven== | ||
| + | [[Image:071107_ayu_gel1.jpg|100px|left]] | ||
| + | [[Image:071107_ayu_gel2.jpg|100px|right]] | ||
| + | * miniprep'd wbbL/neuS funny thing | ||
| + | # restriction map looks funny (see right) | ||
| + | # sent it for sequencing. | ||
| + | * poured super special david plates | ||
| + | * basic parted RFP without start ATG, and plated. | ||
| + | # see picture validating it's coolness on left. | ||
| + | * cleaned my bench and austin bench | ||
| + | ==7/10 another day== | ||
| + | * yfbE w/ and w/o rbs-ATG basic part'd (I716013, I716014) | ||
| + | * did an assay on wbbL/neuS - no lysing occurred with K1 phage. | ||
| + | # growing up for miniprep then sequencing tmrw. | ||
| + | ==7/9 MISSING OLIGOS== | ||
| + | * So my ay013 and ay014 are missing lols, amin will order more ! | ||
| + | * Fresh I716012 plated onto some fresh MC828E. | ||
| + | * i helped austin with some minipreps. | ||
| + | ==7/6 seven-six-oh-seven== | ||
| + | * J1 and J2 are bad, J3 has a high chance of bad | ||
| + | # remaking I716008. digesting J3 in case it's good. | ||
| + | # doesn't look good. Xformed some newly made 716008. | ||
| + | ==7/5 confusing #8== | ||
| + | * digest pcr products 1 and 2 + clean | ||
| + | * make kan plates | ||
| + | * miniprep I716011 | ||
| + | * digest I716008 (it's bad) | ||
| + | * made mC828E | ||
| + | * send I716008 for sequencing | ||
| + | * make LB and agar | ||
| + | ==7/4 needs more firework== | ||
| + | * I716011 | ||
| + | # tryin it again... | ||
| + | * I716012 put in incubator | ||
| + | |||
| + | ==7/3 yfbe new! and other stuff== | ||
| + | * I716012 ptet-rbs-wbbL-rbs-neuS in lo copy | ||
| + | [[Image:070307_ayu_gel1.jpg|100px|right]] | ||
| + | # has issues with digestion. double digests look great, but triple digest still fuzzy | ||
| + | # two ligations of this done, one with double digest (took both fragments) and one with triple (took invisibly place where it should be) | ||
| + | # plated on mc868e that was saved from yesterday OMG I HOPE IT WORKS | ||
| + | * pcr done of yfbE, with ATG on end, and without the ATG or the rbs. | ||
| + | # SEE RIGHT FOR PICTURE (yfbE w/ ATG, w/o stuff, and RFP) | ||
| + | * speaking of RFP, the oligos I made were for a different plasmid RFP. Ohhh boy | ||
| + | ==7/2 july already?== | ||
| + | * I716011 cm-rbs-cytB5-rbs-cytB5red plated | ||
| + | * I716008 ptet/rbs/wbbL/rbs/neuS (EcoRI, BamHI, AlwNI) 2146+1510+553, largest | ||
| + | # overnight digest test because it messed up during the day. | ||
| + | # will put into david plasmid tmrw. | ||
| + | * yfbE primers ordered. also two for getting RFP. | ||
| + | ==6/30 omg weekend rly?== | ||
| + | * miniprep party <del>I716009</del>, I716010, I716008, 9229 Right, 9203 Right, 1090 Left | ||
| + | ==6/29 public market woot== | ||
| + | * yeaa I got up late and didn't do much today. | ||
| + | * cultured some cells from plates, oh boy! | ||
| + | * so boring I didn't even make an agenda .txt file on the comp. | ||
| + | ==6/28 /shruggery== | ||
| + | * incubator at 25 C. wtf. | ||
| + | <del>* 1-2-3ing step 1 now..</del> i forgot to do rbs's. lol. | ||
| + | * xform I716010 (kan+rbs+cytB5red) | ||
| + | * xform I716009 (cm+rbs+cytB5) | ||
| + | * 1-2-3 xform Left 1090 (rbs) | ||
| + | * 1-2-3 xform Right 716005 #G3 (9229) | ||
| + | * 1-2-3 xform Right 716006 #H? (9203) | ||
| + | [[User:Ayu|Ayu]] 21:01, 28 June 2007 (EDT) | ||
| + | |||
| + | ==6/27 growth curve fun== | ||
| + | * Updated registry! yayyyyy | ||
| + | * growth curve on yfbE/rbs/RFP | ||
| + | # :( no phenotype observed for iron thing. | ||
| + | # Sent IB and ID clones of it for sequencing. | ||
| + | # Xformed the aforementioned minipreps into M65 (??) cells that should turn blue (or not?) | ||
| + | * I716008 (Ptet-rbs-wbbL-rbs-neuS) made and xform'd | ||
| + | * incubated Lefty+Righty | ||
| + | |||
| + | ==6/26 dehumidifier machine is still loud== | ||
| + | * Bca9229 and Bca9203 look great (G1, G2, H3, H4) (ay021, 022, 023, 024) | ||
| + | * poured plates | ||
| + | * yfbE works. like 2x. will do growth curve tmrw. | ||
| + | * xformed H4 into Righty, G1+G2 into Lefty | ||
| + | ==6/25 dehumidifier machine is loud== | ||
| + | * Sent G1 and G2 for resequencing, cuz they didn't work. | ||
| + | [[Image:062507_ayu_gel1.jpg|100px|right]] | ||
| + | * test digest yfbE try #2 | ||
| + | # BglII/XhoI | ||
| + | * miniprep 9229 then test digest | ||
| + | # BglII/XhoI - looking for two bands | ||
| + | * 9229 H4,H3 sent for F sequencing w/ ca998 (023,024) | ||
| + | * yfbE incubated in Fe(II)SO4 instead of Fe(III)Cl3. Hope it works! | ||
| + | GEL: h4, h3, h2, iD, iB, iA, ladder | ||
| + | ==6/24 lol weekend== | ||
| + | * incubated yfbE w/ and w/o Fe, and 9229. | ||
| + | ==6/22 floodrly?== | ||
| + | * floodrly? | ||
| + | ==6/21 ok== | ||
| + | [[Image:062107_ayu_gel2.jpg|100px|left]] | ||
| + | [[Image:062107_ayu_gel1.jpg|100px|right]] | ||
| + | * yfbE and neuS didn't work. wbbL was good. | ||
| + | # Chris redoes them all | ||
| + | # and all are plated. yfbE gets extra love with a 20 uL iron plate extra. | ||
| + | * B5 synth'd thing, miniprep'd | ||
| + | # Let's call it I716005 | ||
| + | # looks ok from picture... sending G1 and G3 for forward sequencing. | ||
| + | * Bca9229 - B5 thing, placed into austin digest with BglII/XhoI, xformed | ||
| + | IMGS: (<< Left) The B5 reductase (?) digest looks good.<br> | ||
| + | (>> Right) The digested gel to purify was good. [yfbE, neuS, 1122x3, 1121x3, wbbL]<br> | ||
| + | |||
| + | ==6/20 oops== | ||
| + | [[Image:062007_ayu_gel2.jpg|100px|left]] | ||
| + | * yfbE irony thing... fail | ||
| + | [[Image:062007_ayu_gel1.jpg|100px|right]] | ||
| + | # (BAD) w/ and w/o FeCl3 had no difference | ||
| + | # did mini of the F1, F2, F3 xformed and incubated stuff | ||
| + | # (>> digested mini with EcoRI/BamHI and got the band pattern of the parent vector (1100-1109). So failed xformation. | ||
| + | # I was looking for 3k and a 400, not a 3k and a 1250. | ||
| + | # (FIX) Got good digest of 1100-1109 from Chris, and put with new digest of yfbE to incubate on a plate. | ||
| + | * wbbL and neuS... no colonies on the plate (fail) | ||
| + | # (BAD) I believe I plated wrong. | ||
| + | # <<) A digestion of the miniprep looks fine (so 1121 and 1122 parent plasmids OK) | ||
| + | # And pretty sure that wbbL and neuS were good, and that I cut out bands right. | ||
| + | # (FIX) Redid incubating and plating. | ||
| + | [[User:Ayu|Ayu]] 16:58, 20 June 2007 (EDT) | ||
| + | |||
| + | ==6/19 safety is everyone's job== | ||
| + | * ;-) | ||
| + | * Sequencing received, looks good (ay05,ay06: ay016-18) see seq page | ||
| + | * neuS and wbbL xform'd into 1121 and 1122 libraries. Plated | ||
| + | * F1-4 (yfbE) incushakin, w/ and w/o FeCl3, to test promoter activity | ||
| + | * G1-4 incushakin: B5 (synthesized guy) | ||
| + | <br> | ||
| + | ''random'': woot new fridge! looks quite secksy <3 | ||
| + | ==6/18 speaker party== | ||
| + | * xform'd lotta stuff | ||
| + | * miniprep | ||
| + | * pour plates | ||
| + | * sent wbbL for rev sequencing | ||
| + | * sent HPI/katG for middle sequencing ([ay06] name/ay018 prim/ay007) | ||
| + | <br> | ||
| + | ''other'': set up speaker sys. need M-M cable. woot. | ||
| + | ==6/15 digestion party== | ||
| + | * Good D1, D4 | ||
| + | * synthy plasmid thingy... | ||
| + | # [digest] kristin B4 for backbone. Used EcoRI/XhoI purified L | ||
| + | # [digest] synthy plasmid thingy for insert. Used EcoRI/XhoI purified S | ||
| + | * I716003a (pBca9145- cmr cass+rbs+neuS) | ||
| + | # [digest] pBca9145-neuS (I716001) (BglII, XhoI; 2063+1245; S) | ||
| + | # [digest] pBca1101-I716051 (BamHI, XhoI; 3119+ 850; L) | ||
| + | * pBca9145-yfbE_pro-rbs-RFP (I716004) | ||
| + | # [digest] pBca9145-yfbE_promote (I716002) (EcoRI/BamHI, 2063+ 421, S) | ||
| + | # [digest] pBca1100-Bca1109 (EcoRI/BamHI, 2927+1253, L) | ||
| + | * wbbL | ||
| + | [[Image:061507_ayu_gel1.jpg|100px|right]]<br> | ||
| + | # miniprep'd and ready to go! | ||
| + | # [IMAGE] of gel to the right: E1/E2/E3/E4/ladder >>> | ||
| + | # Sent E1 and E2 for sequencing, forward (ay014, ay015) | ||
| + | <br><br> | ||
| + | ''NOtes'': STILL NEED TO ENTER YFBE PROMOTER PART LOL entering composite parts would be nice too<br> | ||
| + | I did 10 digestions today. I'm proud of myself. | ||
| + | ==6/14 stuff about things== | ||
| + | * neuS clone C1: WE HAVE A WINNER! | ||
| + | * miniprep party, D1/2/3/4 | ||
| + | * digestions didn't work too well mmmm going to sequence D1 and D4. | ||
| + | * wbbL good plate, now incubating in shaker | ||
| + | * cgctattcgcgctacctttg ready to order (middle sequencing of HPI/katG) | ||
| + | ==6/13 gloves, zymo, and ethanol oh my== | ||
| + | * a random day | ||
| + | # neuS digest used to transform n plate new colonies, since the old plate had only 3 people, and 1 which worked | ||
| + | # wbbL digest > new plate as well (old one had one colony and it was bad) | ||
| + | # yfbE put into shakey tubes | ||
| + | * One of the neuS got miniprepped and the test digest looks good compared to test in ApE | ||
| + | [[Image:061307_ayu_gel1.jpg|100px|right]]<br> | ||
| + | # sending it for sequencing, eh. | ||
| + | * Sequencing... | ||
| + | # Most (A1, A4, B1) sucked | ||
| + | # only A3 (HPI/katG) was decent. It might have an addition mutation of a G. | ||
| + | # A3 sent for reverse sequencing with G01001 | ||
| + | * Began redo-ing of HPI/katG-making, with a phase 1 PCR (the halves with a mutation) | ||
| + | <br> | ||
| + | ''Todo'': Input parts in registry (yfbE?) | ||
| + | |||
| + | ==6/12 a bag full of grapes== | ||
| + | * YAY WE ALL GOT OUR OWN SET OF PIPETTE PEOPLE | ||
| + | * PCR of yfbE... | ||
| + | # last night's thing, left in the freezer overnight. >FAIL< | ||
| + | # Did a new PCR -- looks good -- cells xform'd, plate is incubating. | ||
| + | * neuS new xformation looks good. Three colonies now incubating. | ||
| + | * wbbL (1) and HPI/katG (4) | ||
| + | # miniprepped and digest gel ran: | ||
| + | [[Image:061207_ayu_gel1.jpg|100px|right]]<br> | ||
| + | # HPI/katG 1,2,3,4 || wbbL || marker | ||
| + | # 1,2 might be okay.. that faint band is weird. 3 is great! 4 = wtf. wbbL = wtf too (should have two bands) | ||
| + | # decision to put 1,3,4,wbbL for sequencing. | ||
| + | [[User:Ayu|Ayu]] 17:59, 12 June 2007 (EDT) | ||
| + | |||
| + | ==6/11 austin's birthday== | ||
| + | * CAKE PARTY - great custard cake | ||
| + | * I put the wbbL (1) and HPI/katG (4) colonies to incubate in LB broth. | ||
| + | * neuS failed; no colonies :((((((( | ||
| + | ** redid ligation and xformation. hopefully there will be good results tmrw! | ||
| + | * made like 20 LB-Agar/Amp plates - looks like our stock will last at least this week | ||
| + | * researched nitric oxide (NO) and E. Coli - looks like soxRS is promising | ||
| + | * also researched RBCs and how they deal with NO | ||
| + | * plopped yfbE into PCR will do stuff with it tmrw | ||
| + | <br> | ||
| + | ''TO DO'': enter yfbE into the registry <br> | ||
| + | [[User:Ayu|Ayu]] 18:24, 11 June 2007 (EDT) | ||
| + | |||
| + | ==6/8 long day?== | ||
| + | * My PCR from last night (HPI/katG) was ROXOR! (left) | ||
| + | ** xformed some DH10B's. w00t w00t | ||
| + | * Today's PCR was wbbL and neuS. ALSO ROXOR LOL (right) | ||
| + | ** xformed DH10B's. | ||
| + | * made oligos for yfbE promoter thingy - will test with GFP and yeah! next week! | ||
| + | * poured lotsa LB/agar+amp plates | ||
| + | <br> | ||
| + | [[Image:060807_ayu_gel1.jpg|100px|left]] [[Image:060807_ayu_gel2.jpg|100px|right]] | ||
| + | |||
| + | ==6/7 we got benches== | ||
| + | * we got benches | ||
| + | * pcr of [http://partsregistry.org/Part:BBa_I716253:Design HPI/katG from Salmonella] | ||
| + | # well... getting the mutated PCR prod overnight. going to xform tmrw, hope it works! | ||
| + | * programmed pcr on machine upstairs (#6) | ||
| + | * we got computers | ||
| + | * AGAR SUX, for future reference: | ||
| + | # nuke @ 20:00 min, 50% power. | ||
| + | # water bath in tap water for 5-10 min | ||
| + | # thaw the antibiotic right now!! | ||
| + | # FIRE for disinfecting | ||
| + | # pour that stuff. set 15 min, then marker it then bag | ||
| + | |||
| + | ==6/6 waiting for oligos== | ||
| + | * Made oligos and constructs with Vai, for getting wbbL and neuS from pJ23006-Bca9106 | ||
| + | * We tried the P_tet/RFP triple/double digest to make a composite part. | ||
| + | # FAIL | ||
| + | # probably source DNA is bad | ||
| + | # so much for that activity... | ||
| + | <br> | ||
| + | ''Other stuff'': I won speed scrabble. even though I kind of cheated ish (didn't stop when Sam said stop" | ||
| + | |||
| + | ==6/5 coolbeans== | ||
| + | * Finalized oligos to order with Vai | ||
| + | * Learned about LB broth-ing and LB/Agar plating. Thanks, Austin and Sam :) | ||
| + | * Learned about the many composite part-making methods. Props 2 Chris | ||
| + | # prefix/suffix is weaksauce | ||
| + | # Use the AlwnI or BsaI or BglI, in conjuction with BglII or BamHI << (Did this today) | ||
| + | # DBBS | ||
| + | # 3 antibiotic; MIT endorses, used for BioBrick 1.0. Triple digest = bad | ||
| + | # 1-2-3 method << 'Our Goal' in a few weeks. should be leet. | ||
| + | * Planned and vicariously did the making of '''P_tet+RFP''' brick (see [[Vaibhavi_Umesh_Notebook | Vai Notebook]]) | ||
| + | <br> | ||
| + | ''Other Notes:'' All oligos are being ordered, w00t w00t. | ||
| + | [[User:Ayu|Ayu]] 18:36, 5 June 2007 (EDT) | ||
| + | <br> | ||
| + | ==6/4 Training Finishes, Real Stuff Starts== | ||
| + | * Incubated some colonies | ||
| + | * Miniprep'd already-been-incubated colonies (2) | ||
| + | * Double digest of the 2 minipreps + parent plasmid | ||
| + | * Colony PCR'd the incubated E.coli | ||
| + | * Ran gel of the digest + PCR | ||
| + | [[Image:060407gelayu.jpg|200px|right]] | ||
| + | * >>> PCR product / Miniprep 1 / Parent Plasmid / Miniprep 2 / Ladder >>> | ||
| + | * No bands for PCR or parent. Confused? Other ones look great. | ||
| + | <br> | ||
| + | ''As for me:'' Wiki acc works now.<br> Designing oligos and will compare with Vai. | ||
| + | [[User:Ayu|Ayu]] 18:19, 4 June 2007 (EDT) | ||
=== === | === === | ||
<span style='font-family:"Courier New";color:#3366FF;font-size:6.0pt'> | <span style='font-family:"Courier New";color:#3366FF;font-size:6.0pt'> | ||
to do | to do | ||
</span> | </span> | ||
Latest revision as of 23:27, 15 August 2007
My Construction Files
My Sequencing Files
8/15 so Zinc represses growth
- it represses growth! oh nooooo
- will leave assay things going overnight
- retrasnsformed I716034 because i am suspicious of the -80 pir116 i made earlier
8/14 hurrah
- I716034 "knockin'" is xformed into pir116 and plated. hopefully it works.
- so this pzhuC thing works with 20x the zinc used before (i think you need 20 uL of 1M ZnCl2 per each 1 mL)
- growth curve assay says... the things with zinc stopped growth at 0.06 OD. what?????
- incubating various 5 mL tubies of varying zinc added.
8/13 status report
- pzhuC: 8 different clones stuck with zinc to see if any turn off zincyness
- also 30.la and 30.2b put into varying concentrations Zn2+
- wbbl/neus into genome: incubated some clones and will xform tmrw. made competant pir116s today (too lightly colored solution?)
todo: catelogue my boxes. make presentation draft draft. make t-shirts/other clothing
8/7 good productivity
- so what have I done recently?
- I716026 pznuC (Zn2+ repressed promoter) and I716030 composite with RFP. The RFP is incubating right now
- I716028 pmgrB (Mg2+ repressed promoter) and I716031 w/RFP this is where I try to cut off the rbs to just get a basic promoter part.
- I716028 pmgrB and I716032 also incubating. This is where I try to inclube the RBS. Stops before the ORF in the MG1655 genome.
- wbbL neuS - assay worked great, so my clone of I716023 is good (however it's Bbriock v1.0). Currrently working on integrating it into the genome. Just transformed into DH10B today, and plated.
Other things: two bottles of LB went bad (???). somebody jacked my red pipetter. we need more lb/amp plates ._.
8/3 dry ice dry ice dry ice dry ice
- 27 28 29 plated
- wbbL/neuS: incubating. ON culture will be used tmrw in assay.
- plates
8/1 AUG 1 '07 telebears pt 2
- I716023 miniprepped and transformed into MC828 for O antigen testing
- I716027 picked 4 guys and incubating
- PCR did before leaving, for znuC and mgrB promoters.
7/31 my green pipette is missing
- wbbL/neuS assay on I716023 (w/ and w/o K1 phage)
- one clone worked! it's the lysed one in the following picture
- made oligos for
- Vitreosilla sp. VHb hemoglobin
- zinc promoter
- Mg promoter
7/30 vitreoscilla rly?
- Made I716023, which is the wbbL neuS biobrick 1.0 style.
- did some reading to find promoter for sam
7/26 still trying
- I716020 repeat creation is funny, not white. colony pcr says...
- I716008 sent for reverse sequencing to get a complete picture (is it really good? am I just messing up in making I716012?)
7/25 few have won
- I716020 not successfully made. none were red, and the E/Ba digest gel looked really really funny (4.7k, 3k, 2k; expected was 4.9k, 0.9k)
- it was religated and we will see tomorrow how the plate is
- I716012 lol k put in incushaker tubes, we will see tomorrow how the tubes is
7/24 many will enter few will win
- There was one clone of the I716008 that looked good: R4
- a test digest suggests that it is 100% correct
- But the triple digest, while having a 2150 band that I wanted, seemed to only have one 1100 or 900 band. Weird
- transformed into MC828 and MC828E anyway, hopefully it works tmrw
7/23 another day
- iron "UCB" "IGEM 07" redone
- I716008 9 minipreps done, each a diff clone, and sent for forward sequencings.
- I716020 plated.
7/20 Untitled 2
- I716019 found to be created correctly. (t7-rbs-cytB5-rbs-cytB5red-dblterm) see ay42 and ay43
7/19 Untitled
- so the sequencing for wbbL/neuS showed up really mixy and funny. Seems like 1/8 to 1/4 of the library has success though, so I will replate and then mini individual colonies and sequence each one til I find a winner.
- yfbE assay was a success. Crisis averted by finding "Laser On" option in FlowCyto program.
- cytB5/red cassette completed and sent to quintara for sequencing.
7/18 A's take Rangers to school
- A's mediocre, Rangers pretty bad
- I put 12 into the wrong cell >_< well sequencing shows that I'm missing half the promoter anyway so... meh
- yfbE iron promoter workses (P series). See pic on right.
7/17 yaeyeayea
- iron stuff growing tubes. 16 tubes, 4+4 clones jp style and not
- 12 and 19 are growing plate.
7/16 four ligations
- the sequencing for wbbL/neuS library seems like Ptet is cut out. bleh doing again
- made I716008, I716018, I716017, I716016 and plated
7/13 friday
- the RFP without ATG looks good (sequencing)
- wbbl/neuS lib didn't work again hmm, sent for sequencing
- other stuff will insert later
7/12 efficiency
- got news that I716012 didn't sequence well, trying amplify
- incubated I716015 (pBca9145-RFP_noATG)
- miniprepped yfbE's (I716013 and I716014)
- plated proprietary I716016 on FeCl3 and without
- new yfbE stuff for sequencing
- running gel on I716015 pcr prod to expediate testing
- redo I716012 again
- digesting 101 and 008
- growing up electrocompetant MC828E
remarks austin said cytochrome b5 /b5 red was pretty. yeayeayeayaeyea
7/11 seven eleven
- miniprep'd wbbL/neuS funny thing
- restriction map looks funny (see right)
- sent it for sequencing.
- poured super special david plates
- basic parted RFP without start ATG, and plated.
- see picture validating it's coolness on left.
- cleaned my bench and austin bench
7/10 another day
- yfbE w/ and w/o rbs-ATG basic part'd (I716013, I716014)
- did an assay on wbbL/neuS - no lysing occurred with K1 phage.
- growing up for miniprep then sequencing tmrw.
7/9 MISSING OLIGOS
- So my ay013 and ay014 are missing lols, amin will order more !
- Fresh I716012 plated onto some fresh MC828E.
- i helped austin with some minipreps.
7/6 seven-six-oh-seven
- J1 and J2 are bad, J3 has a high chance of bad
- remaking I716008. digesting J3 in case it's good.
- doesn't look good. Xformed some newly made 716008.
7/5 confusing #8
- digest pcr products 1 and 2 + clean
- make kan plates
- miniprep I716011
- digest I716008 (it's bad)
- made mC828E
- send I716008 for sequencing
- make LB and agar
7/4 needs more firework
- I716011
- tryin it again...
- I716012 put in incubator
7/3 yfbe new! and other stuff
- I716012 ptet-rbs-wbbL-rbs-neuS in lo copy
- has issues with digestion. double digests look great, but triple digest still fuzzy
- two ligations of this done, one with double digest (took both fragments) and one with triple (took invisibly place where it should be)
- plated on mc868e that was saved from yesterday OMG I HOPE IT WORKS
- pcr done of yfbE, with ATG on end, and without the ATG or the rbs.
- SEE RIGHT FOR PICTURE (yfbE w/ ATG, w/o stuff, and RFP)
- speaking of RFP, the oligos I made were for a different plasmid RFP. Ohhh boy
7/2 july already?
- I716011 cm-rbs-cytB5-rbs-cytB5red plated
- I716008 ptet/rbs/wbbL/rbs/neuS (EcoRI, BamHI, AlwNI) 2146+1510+553, largest
- overnight digest test because it messed up during the day.
- will put into david plasmid tmrw.
- yfbE primers ordered. also two for getting RFP.
6/30 omg weekend rly?
- miniprep party
I716009, I716010, I716008, 9229 Right, 9203 Right, 1090 Left
6/29 public market woot
- yeaa I got up late and didn't do much today.
- cultured some cells from plates, oh boy!
- so boring I didn't even make an agenda .txt file on the comp.
6/28 /shruggery
- incubator at 25 C. wtf.
* 1-2-3ing step 1 now.. i forgot to do rbs's. lol.
- xform I716010 (kan+rbs+cytB5red)
- xform I716009 (cm+rbs+cytB5)
- 1-2-3 xform Left 1090 (rbs)
- 1-2-3 xform Right 716005 #G3 (9229)
- 1-2-3 xform Right 716006 #H? (9203)
Ayu 21:01, 28 June 2007 (EDT)
6/27 growth curve fun
- Updated registry! yayyyyy
- growth curve on yfbE/rbs/RFP
- :( no phenotype observed for iron thing.
- Sent IB and ID clones of it for sequencing.
- Xformed the aforementioned minipreps into M65 (??) cells that should turn blue (or not?)
- I716008 (Ptet-rbs-wbbL-rbs-neuS) made and xform'd
- incubated Lefty+Righty
6/26 dehumidifier machine is still loud
- Bca9229 and Bca9203 look great (G1, G2, H3, H4) (ay021, 022, 023, 024)
- poured plates
- yfbE works. like 2x. will do growth curve tmrw.
- xformed H4 into Righty, G1+G2 into Lefty
6/25 dehumidifier machine is loud
- Sent G1 and G2 for resequencing, cuz they didn't work.
- test digest yfbE try #2
- BglII/XhoI
- miniprep 9229 then test digest
- BglII/XhoI - looking for two bands
- 9229 H4,H3 sent for F sequencing w/ ca998 (023,024)
- yfbE incubated in Fe(II)SO4 instead of Fe(III)Cl3. Hope it works!
GEL: h4, h3, h2, iD, iB, iA, ladder
6/24 lol weekend
- incubated yfbE w/ and w/o Fe, and 9229.
6/22 floodrly?
- floodrly?
6/21 ok
- yfbE and neuS didn't work. wbbL was good.
- Chris redoes them all
- and all are plated. yfbE gets extra love with a 20 uL iron plate extra.
- B5 synth'd thing, miniprep'd
- Let's call it I716005
- looks ok from picture... sending G1 and G3 for forward sequencing.
- Bca9229 - B5 thing, placed into austin digest with BglII/XhoI, xformed
IMGS: (<< Left) The B5 reductase (?) digest looks good.
(>> Right) The digested gel to purify was good. [yfbE, neuS, 1122x3, 1121x3, wbbL]
6/20 oops
- yfbE irony thing... fail
- (BAD) w/ and w/o FeCl3 had no difference
- did mini of the F1, F2, F3 xformed and incubated stuff
- (>> digested mini with EcoRI/BamHI and got the band pattern of the parent vector (1100-1109). So failed xformation.
- I was looking for 3k and a 400, not a 3k and a 1250.
- (FIX) Got good digest of 1100-1109 from Chris, and put with new digest of yfbE to incubate on a plate.
- wbbL and neuS... no colonies on the plate (fail)
- (BAD) I believe I plated wrong.
- <<) A digestion of the miniprep looks fine (so 1121 and 1122 parent plasmids OK)
- And pretty sure that wbbL and neuS were good, and that I cut out bands right.
- (FIX) Redid incubating and plating.
Ayu 16:58, 20 June 2007 (EDT)
6/19 safety is everyone's job
- ;-)
- Sequencing received, looks good (ay05,ay06: ay016-18) see seq page
- neuS and wbbL xform'd into 1121 and 1122 libraries. Plated
- F1-4 (yfbE) incushakin, w/ and w/o FeCl3, to test promoter activity
- G1-4 incushakin: B5 (synthesized guy)
random: woot new fridge! looks quite secksy <3
6/18 speaker party
- xform'd lotta stuff
- miniprep
- pour plates
- sent wbbL for rev sequencing
- sent HPI/katG for middle sequencing ([ay06] name/ay018 prim/ay007)
other: set up speaker sys. need M-M cable. woot.
6/15 digestion party
- Good D1, D4
- synthy plasmid thingy...
# [digest] kristin B4 for backbone. Used EcoRI/XhoI purified L # [digest] synthy plasmid thingy for insert. Used EcoRI/XhoI purified S
- I716003a (pBca9145- cmr cass+rbs+neuS)
# [digest] pBca9145-neuS (I716001) (BglII, XhoI; 2063+1245; S) # [digest] pBca1101-I716051 (BamHI, XhoI; 3119+ 850; L)
- pBca9145-yfbE_pro-rbs-RFP (I716004)
# [digest] pBca9145-yfbE_promote (I716002) (EcoRI/BamHI, 2063+ 421, S) # [digest] pBca1100-Bca1109 (EcoRI/BamHI, 2927+1253, L)
- wbbL
# miniprep'd and ready to go! # [IMAGE] of gel to the right: E1/E2/E3/E4/ladder >>> # Sent E1 and E2 for sequencing, forward (ay014, ay015)
NOtes: STILL NEED TO ENTER YFBE PROMOTER PART LOL entering composite parts would be nice too
I did 10 digestions today. I'm proud of myself.
6/14 stuff about things
- neuS clone C1: WE HAVE A WINNER!
- miniprep party, D1/2/3/4
- digestions didn't work too well mmmm going to sequence D1 and D4.
- wbbL good plate, now incubating in shaker
- cgctattcgcgctacctttg ready to order (middle sequencing of HPI/katG)
6/13 gloves, zymo, and ethanol oh my
- a random day
- neuS digest used to transform n plate new colonies, since the old plate had only 3 people, and 1 which worked
- wbbL digest > new plate as well (old one had one colony and it was bad)
- yfbE put into shakey tubes
- One of the neuS got miniprepped and the test digest looks good compared to test in ApE
- sending it for sequencing, eh.
- Sequencing...
- Most (A1, A4, B1) sucked
- only A3 (HPI/katG) was decent. It might have an addition mutation of a G.
- A3 sent for reverse sequencing with G01001
- Began redo-ing of HPI/katG-making, with a phase 1 PCR (the halves with a mutation)
Todo: Input parts in registry (yfbE?)
6/12 a bag full of grapes
- YAY WE ALL GOT OUR OWN SET OF PIPETTE PEOPLE
- PCR of yfbE...
- last night's thing, left in the freezer overnight. >FAIL<
- Did a new PCR -- looks good -- cells xform'd, plate is incubating.
- neuS new xformation looks good. Three colonies now incubating.
- wbbL (1) and HPI/katG (4)
- miniprepped and digest gel ran:
- HPI/katG 1,2,3,4 || wbbL || marker
- 1,2 might be okay.. that faint band is weird. 3 is great! 4 = wtf. wbbL = wtf too (should have two bands)
- decision to put 1,3,4,wbbL for sequencing.
Ayu 17:59, 12 June 2007 (EDT)
6/11 austin's birthday
- CAKE PARTY - great custard cake
- I put the wbbL (1) and HPI/katG (4) colonies to incubate in LB broth.
- neuS failed; no colonies :(((((((
- redid ligation and xformation. hopefully there will be good results tmrw!
- made like 20 LB-Agar/Amp plates - looks like our stock will last at least this week
- researched nitric oxide (NO) and E. Coli - looks like soxRS is promising
- also researched RBCs and how they deal with NO
- plopped yfbE into PCR will do stuff with it tmrw
TO DO: enter yfbE into the registry
Ayu 18:24, 11 June 2007 (EDT)
6/8 long day?
- My PCR from last night (HPI/katG) was ROXOR! (left)
- xformed some DH10B's. w00t w00t
- Today's PCR was wbbL and neuS. ALSO ROXOR LOL (right)
- xformed DH10B's.
- made oligos for yfbE promoter thingy - will test with GFP and yeah! next week!
- poured lotsa LB/agar+amp plates
6/7 we got benches
- we got benches
- pcr of [http://partsregistry.org/Part:BBa_I716253:Design HPI/katG from Salmonella]
- well... getting the mutated PCR prod overnight. going to xform tmrw, hope it works!
- programmed pcr on machine upstairs (#6)
- we got computers
- AGAR SUX, for future reference:
- nuke @ 20:00 min, 50% power.
- water bath in tap water for 5-10 min
- thaw the antibiotic right now!!
- FIRE for disinfecting
- pour that stuff. set 15 min, then marker it then bag
6/6 waiting for oligos
- Made oligos and constructs with Vai, for getting wbbL and neuS from pJ23006-Bca9106
- We tried the P_tet/RFP triple/double digest to make a composite part.
- FAIL
- probably source DNA is bad
- so much for that activity...
Other stuff: I won speed scrabble. even though I kind of cheated ish (didn't stop when Sam said stop"
6/5 coolbeans
- Finalized oligos to order with Vai
- Learned about LB broth-ing and LB/Agar plating. Thanks, Austin and Sam :)
- Learned about the many composite part-making methods. Props 2 Chris
- prefix/suffix is weaksauce
- Use the AlwnI or BsaI or BglI, in conjuction with BglII or BamHI << (Did this today)
- DBBS
- 3 antibiotic; MIT endorses, used for BioBrick 1.0. Triple digest = bad
- 1-2-3 method << 'Our Goal' in a few weeks. should be leet.
- Planned and vicariously did the making of P_tet+RFP brick (see Vai Notebook)
Other Notes: All oligos are being ordered, w00t w00t.
Ayu 18:36, 5 June 2007 (EDT)
6/4 Training Finishes, Real Stuff Starts
- Incubated some colonies
- Miniprep'd already-been-incubated colonies (2)
- Double digest of the 2 minipreps + parent plasmid
- Colony PCR'd the incubated E.coli
- Ran gel of the digest + PCR
- >>> PCR product / Miniprep 1 / Parent Plasmid / Miniprep 2 / Ladder >>>
- No bands for PCR or parent. Confused? Other ones look great.
As for me: Wiki acc works now.
Designing oligos and will compare with Vai.
Ayu 18:19, 4 June 2007 (EDT)
to do