Glasgow/Plan
From 2007.igem.org
(→<font face=georgia color=#0000FF size=4>DntR and Dinitrotoluenes</font><br>) |
|||
| (85 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{| valign=top cellpadding=3 | {| valign=top cellpadding=3 | ||
|- | |- | ||
| - | !align=center|[ | + | !align=center|[[Image:Uog.jpg]] || [[Glasgow|<font face=georgia color=#3366CC size=4>Back To <br> Glasgow's <br> Main Page</font>]] |
|} | |} | ||
---- | ---- | ||
| + | |||
| + | {|cellspacing="6px" cellpadding="16" border="0" width="100%" | ||
| + | |- align=center | ||
| + | |||
| + | |[https://2007.igem.org/Glasgow/Wetlab/Goals <font face=georgia color=#3366CC size=5><b>Goals</b></font>] | ||
| + | |[https://2007.igem.org/Glasgow/Wetlab/Results<font face=georgia color=#3366CC size=5><b>Results</b></font>] | ||
| + | |[https://2007.igem.org/Glasgow/Wetlab/Orders <font face=georgia color=#3366CC size=5><b>Sequences</b></font>] | ||
| + | |[https://2007.igem.org/Glasgow/Goals/FuelCells <font face=georgia color=#3366CC size=5><b>Fuel<br>Cells</b></font>] | ||
| + | |[https://2007.igem.org/Glasgow/Wetlab/References <font face=georgia color=#3366CC size=5><b>References</b></font>] | ||
| + | |} | ||
| + | |||
| + | ---- | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>ElectrEcoBlu</font><br> == | ||
| + | |||
| + | [[Image:ElectrEcoBlu.jpg|frame|'''Figure 1:''' Blue pyocyanin produced by ''P. aeruginosa''.]] | ||
| + | |||
| + | ElectrEcoBlu combines an environmental biosensor for common organic pollutants with a microbial fuel cell which can produce its own electricity. These cells produce their own electrical power output which increases in the presence of one or more organic pollutant stimulants. This system has the potential to be used for self-powered long term ''in situ'' and online monitoring with an electrical readout. It is based around novel reporter genes encoding electron carrying mediators which aid the transfer of electrons from the cells to the electrodes resulting in enhanced electricity generation. | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>Environmental Biosensors</font><br> == | ||
| + | |||
| + | A biosensor is “a self contained integrated device consisting of a biological recognition element (enzyme, antibody, receptor or microorganism) which is interfaced to a chemical sensor (i.e., analytical device) that together reversibly respond in a concentration-dependent manner to a chemical species” [https://2007.igem.org/Glasgow/Wetlab/References (Rogers et al, 2006)]. Biosensors offer advantages over current analytical methods for environmental applications such as the possibility of portability and working on-site [https://2007.igem.org/Glasgow/Wetlab/References (Paitan et al, 2003)], and being more cost- and time-effective with the ability of measuring pollutants in complex matrices with minimal sample preparation [https://2007.igem.org/Glasgow/Wetlab/References (Ron, 2007)]. Microorganisms are being developed to exhibit a quick, detectable response to low levels of contamination. These biosensors have the potential to be maintained on-site where they can monitor conditions constantly. For example, biosensors could be used in the soil or water outside of industrial factories to ensure that discharge from the factory is acceptable at all times, or at nuclear reactor sites to make ensure that radioactive materials are not being released into the environment.<br> | ||
<br> | <br> | ||
| + | Conventional reporter genes that have been used in conjunction with biosensors include Green Fluorescent Protein (GFP), firefly/Renilla luciferase and LacZ. All of these have their disadvantages. Although luciferase offers high sensitivity, it also requires the addition of an expensive substrate and a laboratory based assay using expensive equipment. LacZ also requres addition of a substrate and laboratory based assay, and is not as sensitive as luciferase. GFP is readily detectable in the field, but its high stability means that it will build up in the cell over time and thus contribute to background fluorescence. Each of these methods requires skilled workers and expensive equipment. | ||
| - | <center>[[Image: | + | == <font face=georgia color=#0000FF size=4>Mediator Microbial Fuel Cells</font><br> == |
| + | |||
| + | [[Image:Fuelcell.JPG|frame|'''Figure 2:''' Structure of a basic fuel cell. Pyocyanin is a mediator and source of electrons to power the cell.]] | ||
| + | |||
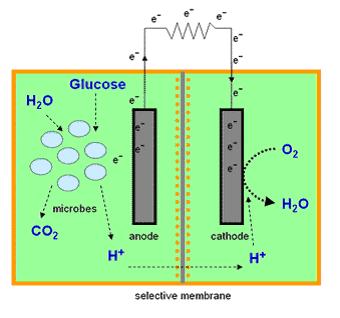
| + | Most microbial cells are electrochemically inactive. The electron transfer from microbial cells to the electrode is facilitated by mediators such as thionine, methyl viologen (methyl blue), neutral red etc, and of the mediators available are expensive and toxic. Microbial fuel cells produce power by use of a microbial cell-permeable chemical mediator, which in the oxidised form intercepts a proportion of NADH (nicotinamide adenine dinucleotide) within the microbial cell and oxidises it to NAD+. The now reduced form of mediator is also cell-permeable and diffuses away from the microbial cell to the anode where, the reduced redox mediator is then electro-catalytically re-oxidised. In addition, cell metabolism produces protons in the anodic chamber, which may migrate through a proton selective membrane to the cathodic chamber. In the latter, they are consumed by ferricyanide (Fe<sup>3-</sup>(CN)<sub>6</sub>) and incoming electrons (via the external circuit) reducing it to ferrocyanide (Fe<sup>4-</sup>(CN)<sub>6</sub>). The oxidised mediator is then free to repeat the cycle. This cycling continually drains off metabolic reducing power from the microbial cells to give electrical power at the electrodes (Figure 2). | ||
| + | |||
| + | [[Glasgow/Goals/FuelCells|See Microbial Fuel Cell Results]] | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>Pyocyanin - A Novel Reporter System</font><br> == | ||
| + | |||
| + | [[Image:Pyocyanin pathway.JPG|frame|'''Figure 3:''' Proposed biosynthetic pathway leading to pyocyanin. (Parsons, 2006).]] | ||
| + | |||
| + | |||
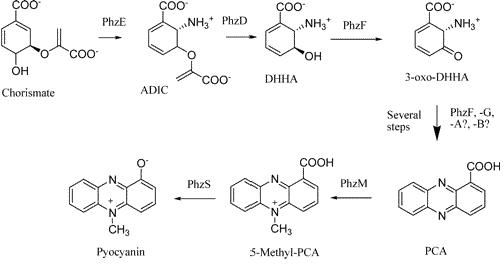
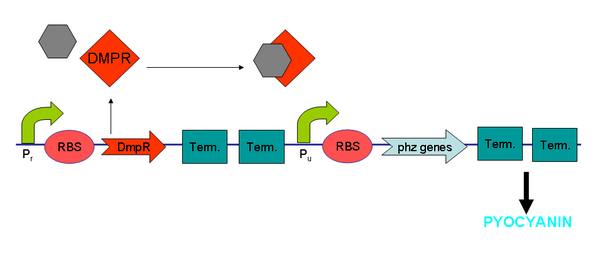
| + | Pyocyanin is a zwitterion synthesised from chorismate by enzymes transcribed from the phz genes in ''Pseudomonas aeruginosa'' PA01. Its synthesis is positively regulated by the LysR-like transcriptional activator MvfR (PqsR) through the synthesis of quorum-sensing quinolone molecules. Pyocyanin engages in oxidation-reduction reactions which deplete cells of NADH, glutathione, and other antioxidants, and produces oxidants such as superoxide and peroxides [https://2007.igem.org/Glasgow/Wetlab/References (Parsons, 2006)]. By isolating the genes phzM, phzS and the seven gene operon phzABCDEFG which express pyocyanin in ''P. aeruginosa'' (Figure 3), we intend to harness the oxidation-reduction potential of pyocyanin to power a microbial fuel cell (Figure 4). While using ''P. aeruginosa'' in microbial fuel cells is extremely promising in fully exploiting and enhancing this technology, it is clearly necessary to either identify or engineer nonpathogenic bacteria that produce similar redox mediators. | ||
| + | |||
| + | We have attempted to clone the pyocyanin producing genes from ''P. aeruginosa'' into ''E. coli'' to harvest pyocyanin and therefore electricity from a non-pathogenic organism. | ||
| + | |||
| + | [[Glasgow/Wetlab/Results#Pyocyanin|See Pyocyanin Results]] | ||
| + | |||
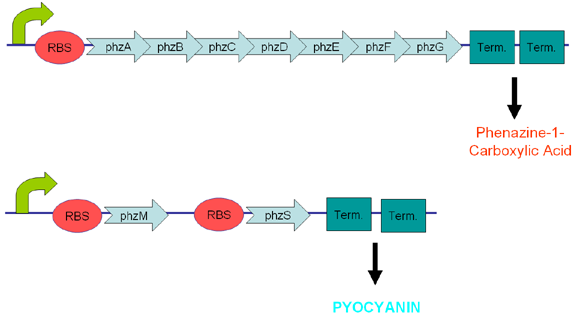
| + | <center>[[Image:Pyo.PNG|600px|frame|'''Figure 4:''' The original phz constructs we designed.]]</center> | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>Bacterial Degradation of Organic Pollutants</font><br> == | ||
| + | |||
| + | Many different species of soil- and water-borne bacteria have adapted to the presence of xenobiotic organic molecules in their environment by developing the capacity to use such compounds as carbon sources. These microbes have evolved networks of enzymes by which complex organic compounds are broken down into metabolic intermediates. In many instances, transcription of the genes encoding the enzymes that participate in these degradation pathways is regulated so that expression of these catabolic enzymes is dramatically enhanced by the presence of the compounds that they degrade. This type of transcriptional control is achieved by the interaction of transcriptional activator proteins with specific gene promoters. These proteins contain a DNA binding domain, a transcriptional activation domain, and a recognition domain. Organic molecules bind to the recognition domain and induce a conformational change in the protein that results in enhanced interaction of the DNA binding domain with specific promoter sequences. The complex effectively initiates transcription of the genes encoding the catabolic enzymes that lie directly downstream of the promoter sequence. | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>XylR and BTEX Chemicals</font><br> == | ||
<br> | <br> | ||
| + | Benzene, Toluene, Ethylbenzene and Xylenes (BTEX chemicals) are types of volatile organic hydrocarbons found in petroleum derivatives and are used extensively in a wide variety of manufacturing processes. Contamination with BTEX chemicals (so named because they are often found together) is typically located near petroleum and natural gas production sites, and in areas with ground storage tanks. | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
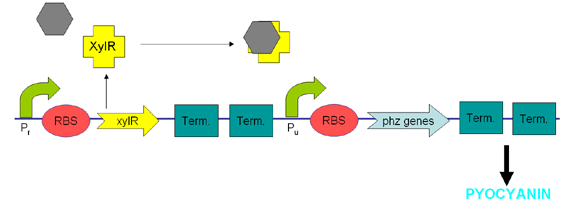
| + | XylR protein, which has evolved in water- and soil-borne bacteria, alters in shape when bound to toluene-like chemicals and forms a transcriptional activator which binds to promoter Pu triggering a cascade of metabolic reactions [https://2007.igem.org/Glasgow/Wetlab/References (Willardson et al, 1998)]. We intend to use XylR and related promoters Pr and Pu to produce pyocyanin in the presence of toluene (Figure 5). | ||
| + | |||
| + | [[Glasgow/Wetlab/Results#XylR and BETX Chemical|See XylR and BTEX Chemicals Results]] | ||
| + | |||
| + | <center>[[Image:Pyo2.png|frame|'''Figure5:''' The XylR construct we designed.]]</center> | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>DntR and Dinitrotoluenes</font><br> == | ||
| + | |||
| + | Dinitrotoluene (DNT) is an intermediate in the commercial production of TNT and is often found as an environmental contaminant in areas surrounding production sites. Being toxic and carcinogenic, it is a pollutant of major concern and therefore previous works have been undertaken to characterize the DNT recognition and degradation operons of Burkholderia cepacia strain R34 – an isolate found to be able to utilise 2,4-DNT as its sole carbon source [https://2007.igem.org/Glasgow/Wetlab/References (R.J. Spanggord 1991)]. During the course of our project we attempted to sub-clone the initial regulatory part of these pathways from a Pseudomonas strain carrying the relevant operon (Rosser & Bruce) with the intention of using it as the sensing component of our biosensor. | ||
| + | |||
| + | The regulatory region consists of a putative LysR-type transcription factor (DntR) consisting of an N-terminal DNA-binding domain and a C-terminal cofactor-binding domain, 301 amino acids in length [https://2007.igem.org/Glasgow/Wetlab/References (Johnson 2002)] which recognises 2,4-DNT and positively regulates transcription of an operon encoding components of the 2,4-DNT dioxygenase complex. DntR also detects salicylate, an intermediate in the metabolic breakdown of polycyclic aromatic hydrocarbons (PAH). These compounds are found as contaminants in the ground surrounding industrial factories that deal with petroleum derivatives. We designed primers to the 3’ end of DntR and to the region immediately upstream of the first ORF in the operon (Figure 6) resulting in a product that conveniently contained both DntR and its target promoter. | ||
| + | |||
| + | [[Glasgow/Wetlab/Results#DntR and Dinitrotoluenes|See DntR and Dinitrotoluenes Results]] | ||
| + | |||
| + | <center>[[Image:Pyo3.png|frame|'''Figure 6:'''The DntR construct we designed.]]</center> | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>DmpR and Phenolic Compounds</font><br> == | ||
| + | |||
| + | DmpR is a transcriptional activator that is specific for phenol and its derivatives (Figure 7). Phenol and cresols are common toxic environmental pollutants. These compounds enter the environment through processes such as paper pulping and milling. Previous works have been undertaken to characterize the phenol recognition and degradation operons of ''Pseudomonas'' sp. strain CF600 – an isolate found to be able to catabolise phenols and cresols [https://2007.igem.org/Glasgow/Wetlab/References (Shingler et al. 1993, Shingler and Moore, 1994)]. | ||
| + | |||
| + | DmpR is a transcriptional activator that is specific for phenol and its derivatives. The 67-kDa dmpR gene product alone was shown to be sufficient for activation of transcription from the dmp operon promoter. Nucleotide sequence determination revealed that DmpR belongs to the NtrC family of transcriptional activators that regulate transcription from -24, -12 promoters. It was also shown that the amino-terminal region of DmpR shared 64% identity with the amino-terminal region of XylR, which is also a member of this family of activators. | ||
| + | |||
| + | [[Glasgow/Wetlab/Results#DmpR and Phenolic Compounds|See DmpR and Phenolic Compounds Results]] | ||
| + | |||
| + | <center>[[Image:600px-DmpR2.PNG|frame|'''Figure 7:''' The DmpR construct we designed.]]</center> | ||
| + | |||
| + | == <font face=georgia color=#0000FF size=4>Model Design and Analysis: outline</font><br> == | ||
| + | |||
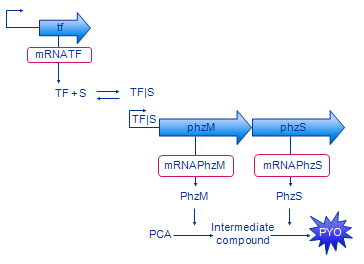
| + | [[Image: Glasgow_design_small.png|frame|Figure 2. Design of our system. The intermediate compound is 5- | ||
| + | methylphenazine-1-carboxylic acid betaine.]] | ||
| + | The biologists constructed an initial diagram to describe the system, using a fairly informal graphical syntax. This used a generic form of the transcription factor ('tf' for the gene, and 'TF' for the protein product) which represented both XylR (BTEX detecting) and DntR (Salicylate detecting). In outline, the steps that we used to develop and refine our model were: | ||
| + | |||
| + | # Simplification by abstracting away the mRNA, thus combining transcription and translation. | ||
| + | # Combining the PhzM and PhzS components to give one step from PCA to PYO | ||
| + | # We also developed a variant of the model with a positive feedback loop | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | [[ | + | We have used a common framework which unifies the qualitative, stochastic and continuous worlds, as a basis for our overall approach to modelling and analysing the biochemical pathways. |
| + | The descriptions (with and without the positive feedback loop) were then transformed into Qualitative Petri Nets (QPN) - see [https://2007.igem.org/Glasgow/Modeling#Petri_Net_Modelling Petri net section]. | ||
| + | We derived the [https://2007.igem.org/Glasgow/Modeling#Parameter_searching_and_refinement rate parameters], and wrote down descriptions and simulated the models in | ||
| + | [https://2007.igem.org/Glasgow/Modeling#Model_design:_detailed Ordinary Differential Equations], as well as in [https://2007.igem.org/Glasgow/Modeling#Petri_Net_Modelling Continuous Petri Nets] (CPN). We performed | ||
| + | model analysis to refine the rate parameters using [https://2007.igem.org/Glasgow/Modeling#Minicap_Sensitivity_Analysis_Program_Package our implementation of the the MPSA algorithm] and [https://2007.igem.org/Glasgow/Modeling#Comparative_Model_Analysis model comparison]. | ||
| + | Simulation of the continuous model was performed in MatLab and [http://www.bionessie.org BioNessie]. | ||
| + | Finally we constructed and simulated | ||
| + | [https://2007.igem.org/Glasgow/Modeling#Stochastic_Modelling stochastic models] in MatLab and | ||
| + | [https://2007.igem.org/Glasgow/Modeling#Stochastic_Modelling_vs._ODE_Modelling compared the results] of from the continuous (ODE-based) and stochastic approaches. | ||
Latest revision as of 17:19, 26 October 2007
 | Back To Glasgow's Main Page |
|---|
| Goals | Results | Sequences | Fuel Cells | References |
Contents |
ElectrEcoBlu
ElectrEcoBlu combines an environmental biosensor for common organic pollutants with a microbial fuel cell which can produce its own electricity. These cells produce their own electrical power output which increases in the presence of one or more organic pollutant stimulants. This system has the potential to be used for self-powered long term in situ and online monitoring with an electrical readout. It is based around novel reporter genes encoding electron carrying mediators which aid the transfer of electrons from the cells to the electrodes resulting in enhanced electricity generation.
Environmental Biosensors
A biosensor is “a self contained integrated device consisting of a biological recognition element (enzyme, antibody, receptor or microorganism) which is interfaced to a chemical sensor (i.e., analytical device) that together reversibly respond in a concentration-dependent manner to a chemical species” (Rogers et al, 2006). Biosensors offer advantages over current analytical methods for environmental applications such as the possibility of portability and working on-site (Paitan et al, 2003), and being more cost- and time-effective with the ability of measuring pollutants in complex matrices with minimal sample preparation (Ron, 2007). Microorganisms are being developed to exhibit a quick, detectable response to low levels of contamination. These biosensors have the potential to be maintained on-site where they can monitor conditions constantly. For example, biosensors could be used in the soil or water outside of industrial factories to ensure that discharge from the factory is acceptable at all times, or at nuclear reactor sites to make ensure that radioactive materials are not being released into the environment.
Conventional reporter genes that have been used in conjunction with biosensors include Green Fluorescent Protein (GFP), firefly/Renilla luciferase and LacZ. All of these have their disadvantages. Although luciferase offers high sensitivity, it also requires the addition of an expensive substrate and a laboratory based assay using expensive equipment. LacZ also requres addition of a substrate and laboratory based assay, and is not as sensitive as luciferase. GFP is readily detectable in the field, but its high stability means that it will build up in the cell over time and thus contribute to background fluorescence. Each of these methods requires skilled workers and expensive equipment.
Mediator Microbial Fuel Cells
Most microbial cells are electrochemically inactive. The electron transfer from microbial cells to the electrode is facilitated by mediators such as thionine, methyl viologen (methyl blue), neutral red etc, and of the mediators available are expensive and toxic. Microbial fuel cells produce power by use of a microbial cell-permeable chemical mediator, which in the oxidised form intercepts a proportion of NADH (nicotinamide adenine dinucleotide) within the microbial cell and oxidises it to NAD+. The now reduced form of mediator is also cell-permeable and diffuses away from the microbial cell to the anode where, the reduced redox mediator is then electro-catalytically re-oxidised. In addition, cell metabolism produces protons in the anodic chamber, which may migrate through a proton selective membrane to the cathodic chamber. In the latter, they are consumed by ferricyanide (Fe3-(CN)6) and incoming electrons (via the external circuit) reducing it to ferrocyanide (Fe4-(CN)6). The oxidised mediator is then free to repeat the cycle. This cycling continually drains off metabolic reducing power from the microbial cells to give electrical power at the electrodes (Figure 2).
See Microbial Fuel Cell Results
Pyocyanin - A Novel Reporter System
Pyocyanin is a zwitterion synthesised from chorismate by enzymes transcribed from the phz genes in Pseudomonas aeruginosa PA01. Its synthesis is positively regulated by the LysR-like transcriptional activator MvfR (PqsR) through the synthesis of quorum-sensing quinolone molecules. Pyocyanin engages in oxidation-reduction reactions which deplete cells of NADH, glutathione, and other antioxidants, and produces oxidants such as superoxide and peroxides (Parsons, 2006). By isolating the genes phzM, phzS and the seven gene operon phzABCDEFG which express pyocyanin in P. aeruginosa (Figure 3), we intend to harness the oxidation-reduction potential of pyocyanin to power a microbial fuel cell (Figure 4). While using P. aeruginosa in microbial fuel cells is extremely promising in fully exploiting and enhancing this technology, it is clearly necessary to either identify or engineer nonpathogenic bacteria that produce similar redox mediators.
We have attempted to clone the pyocyanin producing genes from P. aeruginosa into E. coli to harvest pyocyanin and therefore electricity from a non-pathogenic organism.
Bacterial Degradation of Organic Pollutants
Many different species of soil- and water-borne bacteria have adapted to the presence of xenobiotic organic molecules in their environment by developing the capacity to use such compounds as carbon sources. These microbes have evolved networks of enzymes by which complex organic compounds are broken down into metabolic intermediates. In many instances, transcription of the genes encoding the enzymes that participate in these degradation pathways is regulated so that expression of these catabolic enzymes is dramatically enhanced by the presence of the compounds that they degrade. This type of transcriptional control is achieved by the interaction of transcriptional activator proteins with specific gene promoters. These proteins contain a DNA binding domain, a transcriptional activation domain, and a recognition domain. Organic molecules bind to the recognition domain and induce a conformational change in the protein that results in enhanced interaction of the DNA binding domain with specific promoter sequences. The complex effectively initiates transcription of the genes encoding the catabolic enzymes that lie directly downstream of the promoter sequence.
XylR and BTEX Chemicals
Benzene, Toluene, Ethylbenzene and Xylenes (BTEX chemicals) are types of volatile organic hydrocarbons found in petroleum derivatives and are used extensively in a wide variety of manufacturing processes. Contamination with BTEX chemicals (so named because they are often found together) is typically located near petroleum and natural gas production sites, and in areas with ground storage tanks.
XylR protein, which has evolved in water- and soil-borne bacteria, alters in shape when bound to toluene-like chemicals and forms a transcriptional activator which binds to promoter Pu triggering a cascade of metabolic reactions (Willardson et al, 1998). We intend to use XylR and related promoters Pr and Pu to produce pyocyanin in the presence of toluene (Figure 5).
See XylR and BTEX Chemicals Results
DntR and Dinitrotoluenes
Dinitrotoluene (DNT) is an intermediate in the commercial production of TNT and is often found as an environmental contaminant in areas surrounding production sites. Being toxic and carcinogenic, it is a pollutant of major concern and therefore previous works have been undertaken to characterize the DNT recognition and degradation operons of Burkholderia cepacia strain R34 – an isolate found to be able to utilise 2,4-DNT as its sole carbon source (R.J. Spanggord 1991). During the course of our project we attempted to sub-clone the initial regulatory part of these pathways from a Pseudomonas strain carrying the relevant operon (Rosser & Bruce) with the intention of using it as the sensing component of our biosensor.
The regulatory region consists of a putative LysR-type transcription factor (DntR) consisting of an N-terminal DNA-binding domain and a C-terminal cofactor-binding domain, 301 amino acids in length (Johnson 2002) which recognises 2,4-DNT and positively regulates transcription of an operon encoding components of the 2,4-DNT dioxygenase complex. DntR also detects salicylate, an intermediate in the metabolic breakdown of polycyclic aromatic hydrocarbons (PAH). These compounds are found as contaminants in the ground surrounding industrial factories that deal with petroleum derivatives. We designed primers to the 3’ end of DntR and to the region immediately upstream of the first ORF in the operon (Figure 6) resulting in a product that conveniently contained both DntR and its target promoter.
See DntR and Dinitrotoluenes Results
DmpR and Phenolic Compounds
DmpR is a transcriptional activator that is specific for phenol and its derivatives (Figure 7). Phenol and cresols are common toxic environmental pollutants. These compounds enter the environment through processes such as paper pulping and milling. Previous works have been undertaken to characterize the phenol recognition and degradation operons of Pseudomonas sp. strain CF600 – an isolate found to be able to catabolise phenols and cresols (Shingler et al. 1993, Shingler and Moore, 1994).
DmpR is a transcriptional activator that is specific for phenol and its derivatives. The 67-kDa dmpR gene product alone was shown to be sufficient for activation of transcription from the dmp operon promoter. Nucleotide sequence determination revealed that DmpR belongs to the NtrC family of transcriptional activators that regulate transcription from -24, -12 promoters. It was also shown that the amino-terminal region of DmpR shared 64% identity with the amino-terminal region of XylR, which is also a member of this family of activators.
See DmpR and Phenolic Compounds Results
Model Design and Analysis: outline
The biologists constructed an initial diagram to describe the system, using a fairly informal graphical syntax. This used a generic form of the transcription factor ('tf' for the gene, and 'TF' for the protein product) which represented both XylR (BTEX detecting) and DntR (Salicylate detecting). In outline, the steps that we used to develop and refine our model were:
- Simplification by abstracting away the mRNA, thus combining transcription and translation.
- Combining the PhzM and PhzS components to give one step from PCA to PYO
- We also developed a variant of the model with a positive feedback loop
We have used a common framework which unifies the qualitative, stochastic and continuous worlds, as a basis for our overall approach to modelling and analysing the biochemical pathways.
The descriptions (with and without the positive feedback loop) were then transformed into Qualitative Petri Nets (QPN) - see Petri net section.
We derived the rate parameters, and wrote down descriptions and simulated the models in
Ordinary Differential Equations, as well as in Continuous Petri Nets (CPN). We performed
model analysis to refine the rate parameters using our implementation of the the MPSA algorithm and model comparison.
Simulation of the continuous model was performed in MatLab and [http://www.bionessie.org BioNessie].
Finally we constructed and simulated
stochastic models in MatLab and
compared the results of from the continuous (ODE-based) and stochastic approaches.