Davidson Missouri W/Gene splitting
From 2007.igem.org
(→A Closer Look) |
(→What is Gene Splitting?) |
||
| (9 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
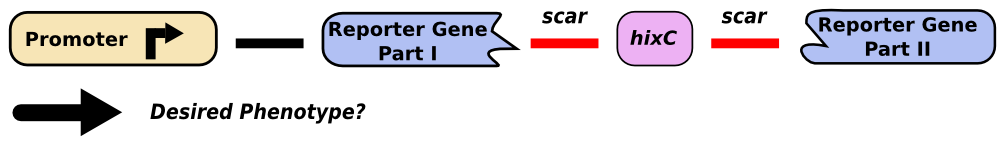
"Gene splitting" refers to the insertion of a ''hixC'' site within the coding region of a gene. Although this allows us to create edges for the simulation of a graph, it will change the protein sequence, potentially interfering with proper functionality. We successfully inserted ''hixC'' in two different reporter genes, GFP and RFP. Cells transformed with plasmids containing these "split" genes still fluoresce the appropriate color. | "Gene splitting" refers to the insertion of a ''hixC'' site within the coding region of a gene. Although this allows us to create edges for the simulation of a graph, it will change the protein sequence, potentially interfering with proper functionality. We successfully inserted ''hixC'' in two different reporter genes, GFP and RFP. Cells transformed with plasmids containing these "split" genes still fluoresce the appropriate color. | ||
| + | |||
| + | [[Image:Unsplit-gene.png|thumb|440px|none|A promoter upstream of a reporter gene will produce the expected phenotype.]] | ||
| + | |||
| + | [[Image:Split-gene.png|thumb|830px|none|The insertion of a ''hixC'' site, or 13 extra amino acids, might prevent the expected phenotype.]] | ||
To facilitate the splitting process we developed software to help us. Our [http://gcat.davidson.edu/iGEM07/genesplitter.html online] gene splitting web tool (click [[Davidson Missouri W/Web tool| here]] for a tutorial) helps us choose PCR primers that will amplify the appropriate segments of a gene of interest. | To facilitate the splitting process we developed software to help us. Our [http://gcat.davidson.edu/iGEM07/genesplitter.html online] gene splitting web tool (click [[Davidson Missouri W/Web tool| here]] for a tutorial) helps us choose PCR primers that will amplify the appropriate segments of a gene of interest. | ||
| Line 17: | Line 21: | ||
|- | |- | ||
| - | |style="color: black; background-color: | + | |style="color: black; background-color: green;" align="center"|GFP - Green Fluorescent Protein |
|In the spring of 2007, Karen Acker pioneered the gene-splitting process by splitting GFP. According to previous research, it had been shown that it was possible to insert extra amino acids within a particular region of the protein without disrupting green fluorescence. Acker successfully inserted a ''hixC'' site at the same location, between amino acids 157 and 158. | |In the spring of 2007, Karen Acker pioneered the gene-splitting process by splitting GFP. According to previous research, it had been shown that it was possible to insert extra amino acids within a particular region of the protein without disrupting green fluorescence. Acker successfully inserted a ''hixC'' site at the same location, between amino acids 157 and 158. | ||
| Line 38: | Line 42: | ||
=A Closer Look= | =A Closer Look= | ||
| - | |||
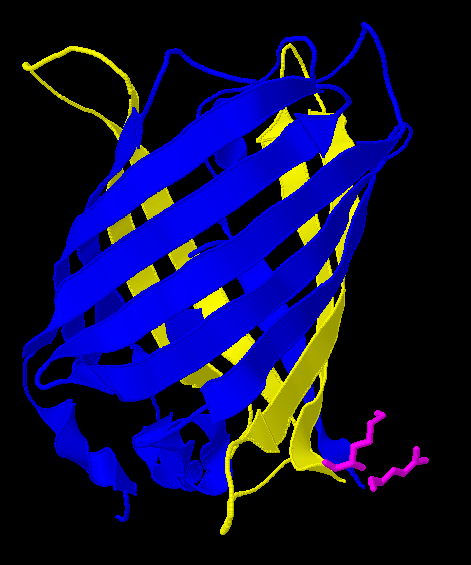
| - | [[Image:Gfp-nosplit.png|thumb|340px| | + | Here is a more detailed view of splitting GFP. The image on the left, with the protein structure highlighted in green, shows the normal, wild-type structure of the protein. The image on the right highlights the two parts of the protein on either side of the ''hixC'' insertion, shown in pink. The additional 13 amino acids are not shown, but they would extend from one pink amino acid into the next. |
| + | |||
| + | From these 3-dimensional PDB images it becomes apparent that the insertion likely sticks into open space away from the rest of the protein. This increases the likelihood that the addition does not interfere with the beta barrel structure or with the chromophore. | ||
| + | |||
| + | As a consequence of RFP's similar 3-dimensional structure it is assumed the ''hixC'' insertion acts in a similar fashion. | ||
| + | |||
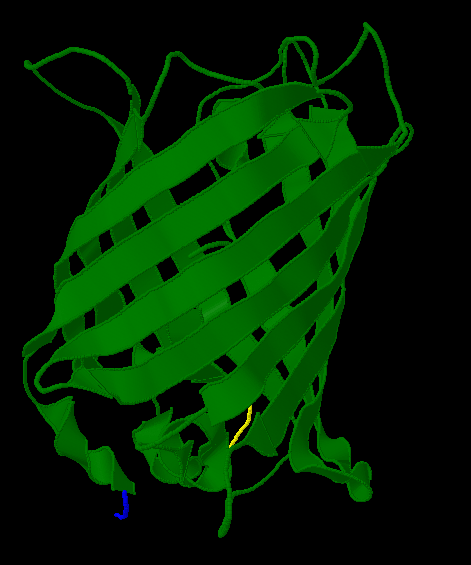
| + | [[Image:Gfp-nosplit.png|thumb|340px|left|The unmodified structure of GFP.]] | ||
| + | [[Image:Gfp-split.png|thumb|340px|none|The sections before and after the ''hixC'' insertion are highlighted in blue and yellow. The amino acids at the insertion point are shown in pink.]] | ||
| + | |||
| + | <br> | ||
| + | <hr> | ||
| + | <center> | ||
| + | <[[Davidson Missouri W/Mathematical Modeling | Previous Section]] | [[Davidson Missouri W/Results | Next Section>]] | ||
| + | </center> | ||
Latest revision as of 18:38, 23 October 2007
What is Gene Splitting?
"Gene splitting" refers to the insertion of a hixC site within the coding region of a gene. Although this allows us to create edges for the simulation of a graph, it will change the protein sequence, potentially interfering with proper functionality. We successfully inserted hixC in two different reporter genes, GFP and RFP. Cells transformed with plasmids containing these "split" genes still fluoresce the appropriate color.
To facilitate the splitting process we developed software to help us. Our [http://gcat.davidson.edu/iGEM07/genesplitter.html online] gene splitting web tool (click here for a tutorial) helps us choose PCR primers that will amplify the appropriate segments of a gene of interest.
The Genes
A Closer Look
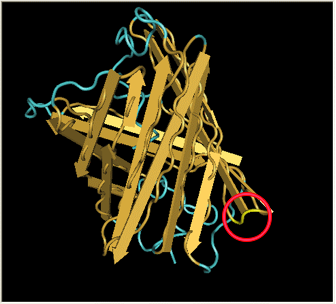
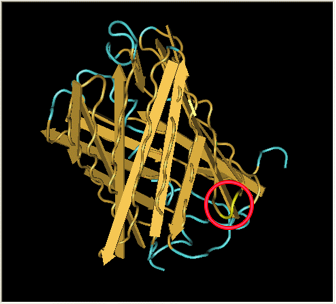
Here is a more detailed view of splitting GFP. The image on the left, with the protein structure highlighted in green, shows the normal, wild-type structure of the protein. The image on the right highlights the two parts of the protein on either side of the hixC insertion, shown in pink. The additional 13 amino acids are not shown, but they would extend from one pink amino acid into the next.
From these 3-dimensional PDB images it becomes apparent that the insertion likely sticks into open space away from the rest of the protein. This increases the likelihood that the addition does not interfere with the beta barrel structure or with the chromophore.
As a consequence of RFP's similar 3-dimensional structure it is assumed the hixC insertion acts in a similar fashion.