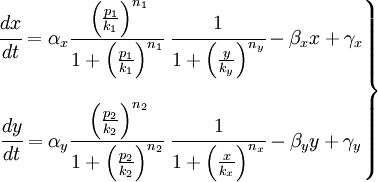Valencia/Promoter calibrator
From 2007.igem.org
(→Design) |
|||
| (8 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
There were several reasons to select that project, but the most important arguments that convince us to perform that project instead of the others were: | There were several reasons to select that project, but the most important arguments that convince us to perform that project instead of the others were: | ||
| - | * It is simple enough to be implemented in the lab in the two months that we have up to | + | * It is simple enough to be implemented in the lab in the two months that we have up to the Jamboree. |
* The calibration of promoters is a prior requirement for developing the projects like the Analog-Digital converter, the filter or the sensor with a graded response. In all those projects, the relative strenght of the initial prometers must be characterized very well. | * The calibration of promoters is a prior requirement for developing the projects like the Analog-Digital converter, the filter or the sensor with a graded response. In all those projects, the relative strenght of the initial prometers must be characterized very well. | ||
| - | * Other tentative projects like the controller, although they | + | * Other tentative projects like the controller, although they appear as very interesting projects, show too much dependence on the fine tunning of the kinetic costants of the promoters in the in silico analysis developed previously. So we decided to discard them because it could take time to fit the system. |
| - | + | We aim at calibrating the parameters of two given promoters. We want to prove it for pOmpR and pOmpRm, but we are confident that this comparator, used as a calibrating device, can be used for as many promoters as one would like. furthermore, we are sure that this is one of the practical uses this comparator has, but with time and will, we could achieve many others... your imagination is the limit! | |
==Design== | ==Design== | ||
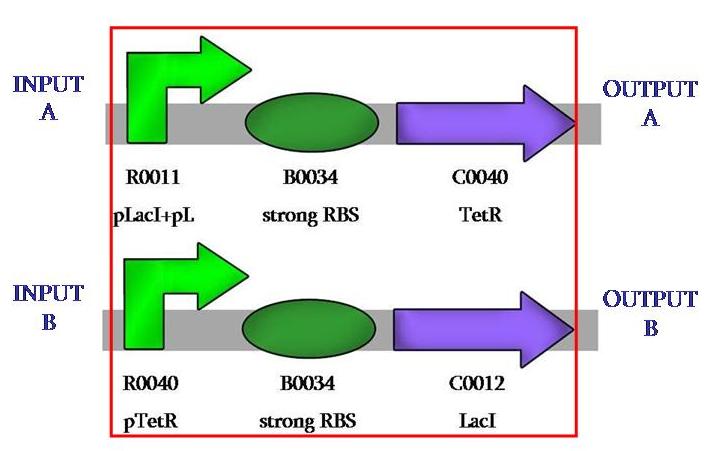
we designed our comparator with a crossed invertion, that act as '''repressor''': protein TetR represses pTetR and LacI does the same with pLacI. | we designed our comparator with a crossed invertion, that act as '''repressor''': protein TetR represses pTetR and LacI does the same with pLacI. | ||
| - | + | we cluster it with two sensing promoters, that act as '''activator'''. those elements drive the different concentrations of the repressor proteins and, thus, the reporter protein too. those effects can be clearly seen on the equations, where ''x'' and ''y'' are the repressor proteins. | |
| - | + | [[Image:VSystempromotores.png|center]] | |
| - | [ | + | In our case, the repressors will always be the same and we will change the activators, the sensing promoters. the biological design was aimed at having the biggest possible modularity. we have succesfully cloned the two parts of our [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Valencia comparator] so to have the possibility of insert whatever one wants to detect at the prefix and whatever one wants to have as output at the sufix. |
| - | + | [[Image:VComparator.jpg|400px|center]] | |
| - | + | ||
| + | |||
| + | <center>'''think modular!'''</center> | ||
==Function== | ==Function== | ||
the calibrator main use is to compare different promoter strenghts. we focused on sensing promoters as they are more easy to implement experimentally. But it is important to note that the input and the output of this device are only limited by our imagination. | the calibrator main use is to compare different promoter strenghts. we focused on sensing promoters as they are more easy to implement experimentally. But it is important to note that the input and the output of this device are only limited by our imagination. | ||
| - | we have started to work with osmolarity detectors pOmpR and pOmpRm as we had some experience with them and we wanted to study their parameters more closely. And we will see our system's behaviour with fluorescence. we have on mind to make as many experiments as we need so to close the parameters values as much as we can. this useful data will come from two kind of experiments: having a constant sensed concentration we will see the dynamics of the fluorescence and varying the sensed concentrations we will see the stable fluorescence values. | + | we have started to work with osmolarity detectors pOmpR and pOmpRm as we had some experience with them and we wanted to study their parameters more closely. And we will see our system's behaviour with fluorescence. we have on mind to make as many experiments as we need so to close the parameters values as much as we can. this useful data will come from two kind of experiments: having a constant sensed concentration we will see the dynamics of the fluorescence and varying the sensed concentrations we will see the stable fluorescence values at the different steady states. |
| + | |||
| + | we want to study the parameters of our equations. starting from the transcripition rate α to the parameters that depend on the repressors. | ||
| + | |||
| - | + | <center>'''Take a look at the [[Valencia/Silico Work|in silico work]] and at what we have done at the [[Valencia/Lab Work| lab]].'''</center> | |
Latest revision as of 14:53, 26 October 2007

|
|
Introduction
From all the ideas we could have implemented from the comparator device we have chosen the promoter calibrator.
There were several reasons to select that project, but the most important arguments that convince us to perform that project instead of the others were:
- It is simple enough to be implemented in the lab in the two months that we have up to the Jamboree.
- The calibration of promoters is a prior requirement for developing the projects like the Analog-Digital converter, the filter or the sensor with a graded response. In all those projects, the relative strenght of the initial prometers must be characterized very well.
- Other tentative projects like the controller, although they appear as very interesting projects, show too much dependence on the fine tunning of the kinetic costants of the promoters in the in silico analysis developed previously. So we decided to discard them because it could take time to fit the system.
We aim at calibrating the parameters of two given promoters. We want to prove it for pOmpR and pOmpRm, but we are confident that this comparator, used as a calibrating device, can be used for as many promoters as one would like. furthermore, we are sure that this is one of the practical uses this comparator has, but with time and will, we could achieve many others... your imagination is the limit!
Design
we designed our comparator with a crossed invertion, that act as repressor: protein TetR represses pTetR and LacI does the same with pLacI.
we cluster it with two sensing promoters, that act as activator. those elements drive the different concentrations of the repressor proteins and, thus, the reporter protein too. those effects can be clearly seen on the equations, where x and y are the repressor proteins.
In our case, the repressors will always be the same and we will change the activators, the sensing promoters. the biological design was aimed at having the biggest possible modularity. we have succesfully cloned the two parts of our [http://partsregistry.org/cgi/partsdb/pgroup.cgi?pgroup=iGEM2007&group=Valencia comparator] so to have the possibility of insert whatever one wants to detect at the prefix and whatever one wants to have as output at the sufix.
Function
the calibrator main use is to compare different promoter strenghts. we focused on sensing promoters as they are more easy to implement experimentally. But it is important to note that the input and the output of this device are only limited by our imagination.
we have started to work with osmolarity detectors pOmpR and pOmpRm as we had some experience with them and we wanted to study their parameters more closely. And we will see our system's behaviour with fluorescence. we have on mind to make as many experiments as we need so to close the parameters values as much as we can. this useful data will come from two kind of experiments: having a constant sensed concentration we will see the dynamics of the fluorescence and varying the sensed concentrations we will see the stable fluorescence values at the different steady states.
we want to study the parameters of our equations. starting from the transcripition rate α to the parameters that depend on the repressors.