Melbourne/Lab Notebook gv 3
From 2007.igem.org
< Melbourne(Difference between revisions)
| Line 46: | Line 46: | ||
* [[Melbourne/Glycerol Stocks|Produced glycerol stocks]] from 900uL of each overnight culture. | * [[Melbourne/Glycerol Stocks|Produced glycerol stocks]] from 900uL of each overnight culture. | ||
* [[Melb:Miniprep protocol|Minipreped remaining culture (4mL) giving 100uL.]] | * [[Melb:Miniprep protocol|Minipreped remaining culture (4mL) giving 100uL.]] | ||
| - | * [[Melbourne/equipement/Measure DNA|Measure DNA concentrations]] using | + | * [[Melbourne/equipement/Measure DNA|Measure DNA concentrations]] using 5uL of miniprep(100uL) diluted in 45uL of TE. |
{|| border="1" | {|| border="1" | ||
| Line 74: | Line 74: | ||
*[[Melbourne/Loading a DNA gel|Loaded]] 20uL of digest | *[[Melbourne/Loading a DNA gel|Loaded]] 20uL of digest | ||
*Digest Pattern | *Digest Pattern | ||
| - | *Expected effects | + | *Expected effects |
| - | + | ||
{| border="1" | {| border="1" | ||
|+ PstI digest | |+ PstI digest | ||
|- | |- | ||
| - | !History!!Mutation tube !!FRAGMENTS | + | !History!!Mutation tube !!FRAGMENTS |
|- | |- | ||
| - | !pNL29T1-(Comp-g318a)-(GvpL-g351a)->!!21|| 5983|| 2516!! 483|| <- | + | !pNL29T1-(Comp-g318a)-(GvpL-g351a)->!!21|| || 5983|| 2516!! 483|| <- |
|- | |- | ||
| - | !pNL29T1-(GvpL-g351a)-(GvpL-g318a)->!!22|| 5983|| 2516!! 483|| <- | + | !pNL29T1-(GvpL-g351a)-(GvpL-g318a)->!!22|| || 5983|| 2516!! 483|| <- |
|- | |- | ||
| - | !pNL26P3-(GvpP-g441a)-(GvpQ-g183a)->!!23!! 4148 || 3170 || 2516 || 378 !! <- || 105 | + | !pNL26P3-(GvpP-g441a)-(GvpQ-g183a)->!!23 || !! 4148 || 3170 || 2516 || 378 !! <- || 105 |
|- | |- | ||
| - | !pNL26P3-(GvpQ-g183a)-(GvpP-g441a)->!!24 !! 4148 || 3170 || 2516 || 378 !! <- || 105 | + | !pNL26P3-(GvpQ-g183a)-(GvpP-g441a)->!!24 || !! 4148 || 3170 || 2516 || 378 !! <- || 105 |
|- | |- | ||
| - | !pNL29T1-(GvpL-g351a)-(GvpL-g696a)->!!25|| 5983!! 2894|| <-|| 105 | + | !pNL29T1-(GvpL-g351a)-(GvpL-g696a)->!!25|| || 5983!! 2894|| <-|| 105 |
|- | |- | ||
| - | !pNL29T1-(GvpL-g351a)-(GvpL-g213a)->!!26!!6088|| 2516|| 378|| <- | + | !pNL29T1-(GvpL-g351a)-(GvpL-g213a)->!!26 || !!6088|| 2516|| 378|| <- |
|- | |- | ||
| - | !pNL26P3-(GvpQ-g183a)-(GvpQ-g150a)->!!27|| 3928 !! 3390 || 2516 || 378 !! <- || 105 | + | !pNL26P3-(GvpQ-g183a)-(GvpQ-g150a)->!!27 || || 3928 !! 3390 || 2516 || 378 !! <- || 105 |
|} | |} | ||
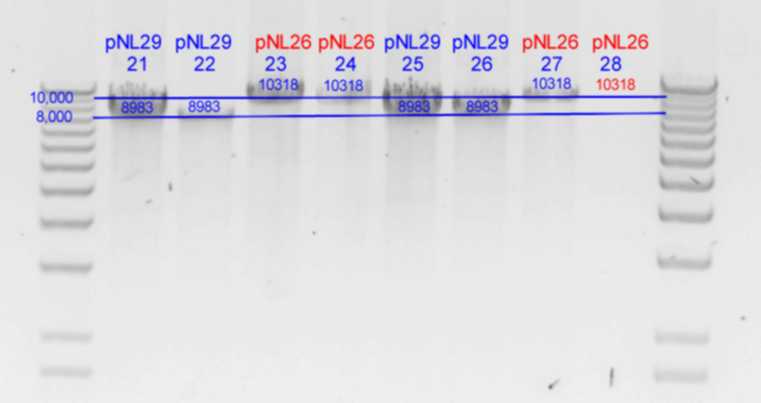
| - | + | *Results of PstI diagnostics:[[Image:Melbourne-gv mut 2p.jpg|right|thumb|850px|PstI digest]] | |
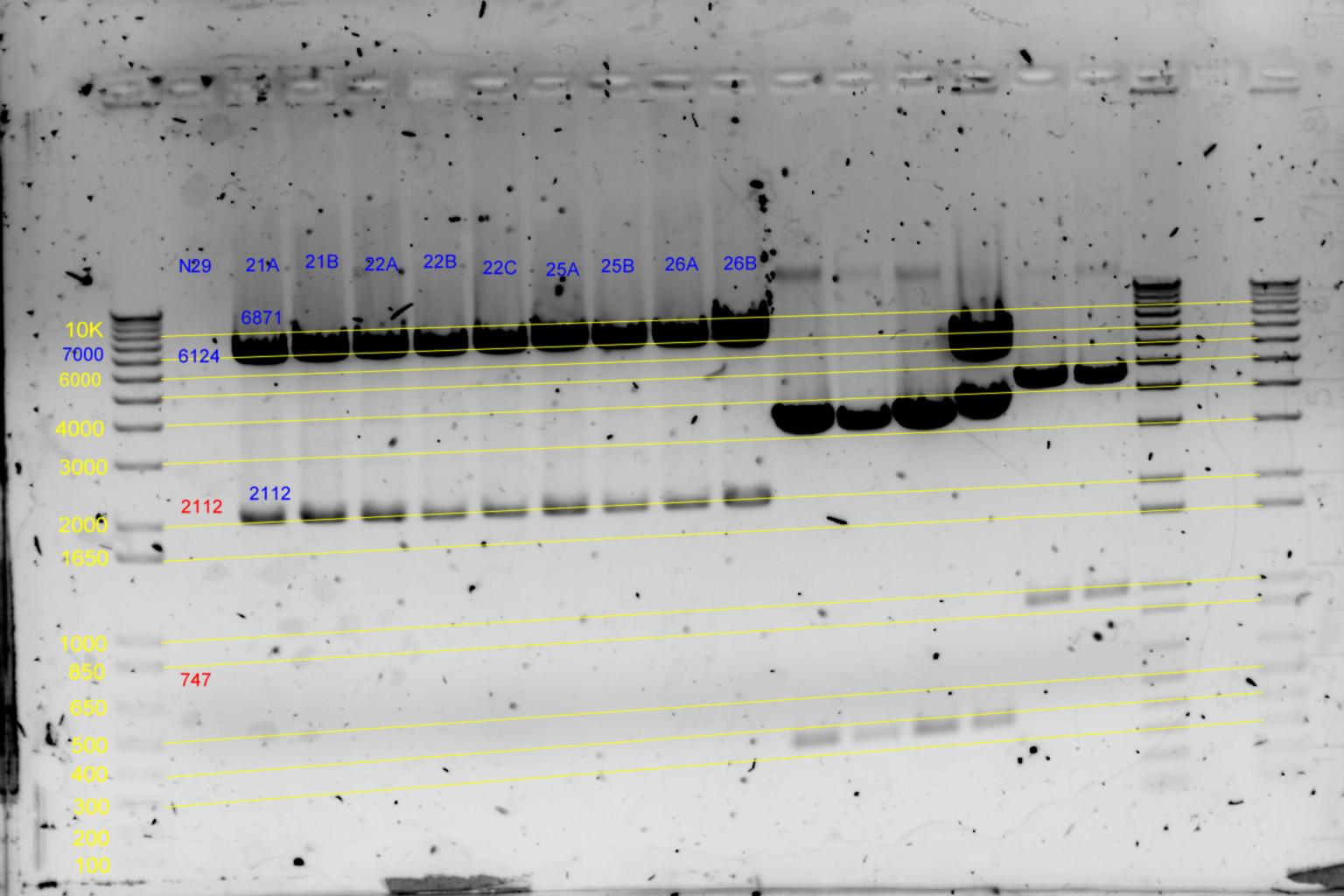
| + | [[Image:Melbourne-gv mut 2e.jpg|left|thumb|350px|EcoRI & BamHI digests]] | ||
{| border="1" | {| border="1" | ||
|+ EcoRI and BamHI digest | |+ EcoRI and BamHI digest | ||
|- | |- | ||
!History!!Mutation tube!!Fragments: | !History!!Mutation tube!!Fragments: | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|- | |- | ||
!pNL29T1-(Comp-g318a)-(GvpL-g351a)->!!21|| !! 6871|| 2112!! <- | !pNL29T1-(Comp-g318a)-(GvpL-g351a)->!!21|| !! 6871|| 2112!! <- | ||
| Line 118: | Line 108: | ||
|- | |- | ||
!pNL29T1-(GvpL-g351a)-(GvpL-g213a)->!!26|| !! 6871|| 2112!! <- | !pNL29T1-(GvpL-g351a)-(GvpL-g213a)->!!26|| !! 6871|| 2112!! <- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| - | * | + | *Results of EcoRI diagnostics: |
| - | + | Conclude 21A,22A,23A are all satisfactory. | |
Latest revision as of 14:22, 25 October 2007
Prepared for site dirrected mutagenesis
- Produced NZY broth 100mL autoclaved (due to contamination in last batch).
- Diluted DNA from last round
| Tube | conc of miniprep ng/ul total=(100ul) | 1uL miniprep added to x uL milliQ |
|---|---|---|
| 5A | 141 | 522 |
| 6A | 212 | 784 |
| 11A | 221 | 817 |
| 10B | 253 | 936 |
Site dirrected Mutagenesis Round #2
- Applied the stratagene Site directed mutagenesis protocolto the following DNA and primer pairs, which when plated out on LB AMP plates producing the numbers of colonies shown. Several of these were picked and tubes marked with a character suffix as shown in table.
Changed PCR conditions on some primers that didn't work before to see if they would work. Specifically segment1 increased from 1 minute @ 95deg to 2minutes, segmet 2 increased from 50 sec @ 95 deg to 1 minute, and elongation time increased from 10 min @ 68 deg to 15 minutes.
| Mutation Number | Template DNA (10ng) | Sence Primer | Antisence Primer | #Colonies | Picks named |
|---|---|---|---|---|---|
| 21 | 5A | GvpL-g351a | GvpL-g351a-R | 21A,21B | |
| 22 | 6A | GvpL-g318a | GvpL-g318a-R | 3 | 22A,22B,22C |
| 23 | 11A | GvpQ-g183a | GvpQ-g183a-R | 26 | 23A,23B |
| 24 | 10B | GvpP-g441a | GvpP-g441a-R | 60 | 24A,24B |
| 25 | 6A | GvpL-g696a | GvpL-g696a-R | 1 | 25A,25B (new PCR conditions) |
| 26 | 6A | GvpL-g213a | GvpL-g213a-R | 26A,26B (new PCR conditions) | |
| 27 | 10B | GvpQ-g150a | GvpQ-g150a-R | 27A,27B (new PCR conditions) | |
| 28 | 10B | GvpQ-g150a | GvpQ-g150a-R | None |
- Ran gel of 10uL sample of pcr reaction with 1uL of bluejuice.
- showed lane GvpQ-g150a non functional in old conditions but functional in new conditions.
- Picked colonies from Plates and culture overnight
- Produced glycerol stocks from 900uL of each overnight culture.
- Minipreped remaining culture (4mL) giving 100uL.
- Measure DNA concentrations using 5uL of miniprep(100uL) diluted in 45uL of TE.
| History | Mutation tube\colony: | A | B | C | D | E |
|---|---|---|---|---|---|---|
| pNL29T1-(Comp-g318a)-(GvpL-g351a)-> | 21 | 183 | ||||
| pNL29T1-(GvpL-g351a)-(GvpL-g318a)-> | 22 | 176 | ||||
| pNL26P3-(GvpP-g441a)-(GvpQ-g183a)-> | 23 | 247 | ||||
| pNL26P3-(GvpQ-g183a)-(GvpP-g441a)-> | 24 | |||||
| pNL29T1-(GvpL-g351a)-(GvpL-g696a)-> | 25 | |||||
| pNL29T1-(GvpL-g351a)-(GvpL-g213a)-> | 26 | |||||
| pNL26P3-(GvpQ-g183a)-(GvpQ-g150a)-> | 27 |
- Diagnostic digests were performed using
- A) 21.5uL of each in Buffer3 with 1uL PstI (20U) at 37degC for 2h30min. (25uL reaction)
- B) 21.5uL of each p29T1 based colony in Buffer2 with 1uL EcoRI (20U) and 1uL BamHI (20U) at 37degC for 2h30min. (26uL reaction)
- C) 21.5uL of each pNL26P3 based colony and in Buffer2 with 1uL EcoRI (20U) at 37degC for 2h30min. (25uL reaction)
- Prepared two 20 lane 100mL agarose gel
- Loaded 20uL of digest
- Digest Pattern
- Expected effects
| History | Mutation tube | FRAGMENTS | ||||||
|---|---|---|---|---|---|---|---|---|
| pNL29T1-(Comp-g318a)-(GvpL-g351a)-> | 21 | 5983 | 2516 | 483 | <- | |||
| pNL29T1-(GvpL-g351a)-(GvpL-g318a)-> | 22 | 5983 | 2516 | 483 | <- | |||
| pNL26P3-(GvpP-g441a)-(GvpQ-g183a)-> | 23 | 4148 | 3170 | 2516 | 378 | <- | 105 | |
| pNL26P3-(GvpQ-g183a)-(GvpP-g441a)-> | 24 | 4148 | 3170 | 2516 | 378 | <- | 105 | |
| pNL29T1-(GvpL-g351a)-(GvpL-g696a)-> | 25 | 5983 | 2894 | <- | 105 | |||
| pNL29T1-(GvpL-g351a)-(GvpL-g213a)-> | 26 | 6088 | 2516 | 378 | <- | |||
| pNL26P3-(GvpQ-g183a)-(GvpQ-g150a)-> | 27 | 3928 | 3390 | 2516 | 378 | <- | 105 |
- Results of PstI diagnostics:
| History | Mutation tube | Fragments: | |||
|---|---|---|---|---|---|
| pNL29T1-(Comp-g318a)-(GvpL-g351a)-> | 21 | 6871 | 2112 | <- | |
| pNL29T1-(GvpL-g351a)-(GvpL-g318a)-> | 22 | 6871 | 2112 | <- | |
| pNL29T1-(GvpL-g351a)-(GvpL-g696a)-> | 25 | 6871 | 2112 | <- | |
| pNL29T1-(GvpL-g351a)-(GvpL-g213a)-> | 26 | 6871 | 2112 | <- |
- Results of EcoRI diagnostics:
Conclude 21A,22A,23A are all satisfactory.