Tianjin/DIODE/Experiment1
From 2007.igem.org
Sunlovedie (Talk | contribs) m |
Lovecarrot (Talk | contribs) |
||
| (10 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
| - | + | Because the AHL signal has to be transferred between different kinds of cell which must be separately cultured according to the principles of our design,we decide to immobilize the generator cell(BBa_I13202),amplifier cells(BBa_I15030),block cells(BBa_I13208) using alginate sodium. | |
| - | + | Another advantage of immobilizing the amplifier cell is that we want to control the positive feedback which acted as Quorum Sensing in nature by input AHL molecular to cause an input decided system from a time-decided QS system.<br><br> | |
| - | Another | + | After immobilizing these cells ,we test them by samplling the culture medium of the tested cells at different time and mixing it together with detector cell for 1.5h to measure the fluorescence intensity. All the fluorescence intensity is quantificationally measured by Virian Cary Eclipse fluorescence spectrophotometer using the 3ml cup at following condition : Excitation wavelength-501nm(5nm),Emission wavelength-511nm(5nm),Average time 0.5s.<br> |
| + | <br> | ||
| + | The function of the generator and block cell is the same as what we expect but the function of the amplifier cells is still waiting to be improved. As our experiment discovered, the amplifier cells can produce a lot of AHL because of the induction of leakage expression of AHL by themselves, so the positive feedback permanently exists because there is no difference between the AHL generating rate of the amplifiers with or without AHL added at the beginning.So we speculate that we have immobilized the cells whose expression of LuxI gene is persistent and can not be inhibited because of the abundant AHL in the culture and in the alginate sodium beads which is hard to eliminate. Then we tried to treat these alginate sodium beads with sodium hydroxide in which the AHL will decompose more quickly .We tested it under various conditions but only to find that the addition of sodium hydroxide is toxic to cells and severely damages its metabolic activities ,so we have to abandon this method to improve the performance of amplifier cells. | ||
| + | <br><br> | ||
| + | Then we cut off the function part BBa_I13202 to ligate it with another vector pSB2K3 whose copy number is controlled by IPTG hoping that after induction of IPTG simultaneously with AHL could boost the duplication of functional part and thus to produce far more AHL than self-induced cells. The experimental results indicate that the performance of amplifier cells has been ameliorated a little bit.<br><br> | ||
| + | |||
==Test result== | ==Test result== | ||
| + | All the result of fluorescence values is based on the function of our detector cell which is shown [[Tianjin/DIODE/Experiment|here]].<br> | ||
| Line 9: | Line 15: | ||
<center><font size="5" color="#0000CC">1.</font><font size="5" color="#0000CC">Function of Block cells</font></center><br> | <center><font size="5" color="#0000CC">1.</font><font size="5" color="#0000CC">Function of Block cells</font></center><br> | ||
| - | Figure 1: AHL tends to be | + | Figure 1: AHL tends to be degraded gradually by immobilized block cells as both figure indicated, after 7 hours, the concentration of AHL has dropped down to less than half of the original value. The difference between the time needed to observe remarkable decline is because the number of immobilized beads on the left figure outnumbered that on the right. Therefore, block cells still work well after being immobilized. |
[[Image:TJU4J1.jpg|400px]] [[Image:TJU4J2.jpg|400px]] | [[Image:TJU4J1.jpg|400px]] [[Image:TJU4J2.jpg|400px]] | ||
<br><br><br> | <br><br><br> | ||
| Line 19: | Line 25: | ||
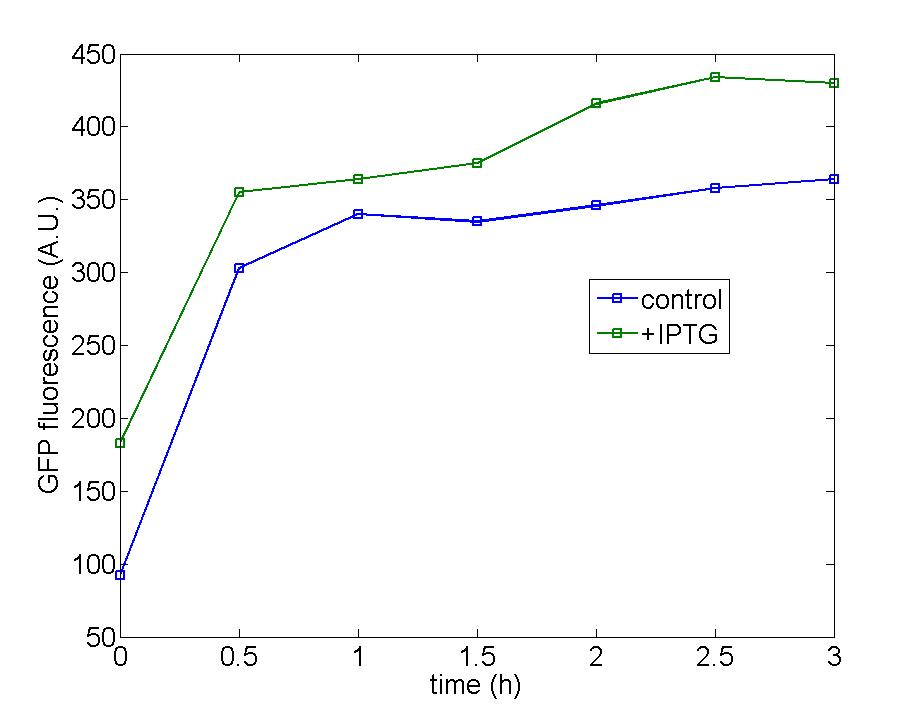
<center><font size="5" color="#0000CC">2.</font><font size="5" color="#0000CC">Function of Generator cells</font></center><br> | <center><font size="5" color="#0000CC">2.</font><font size="5" color="#0000CC">Function of Generator cells</font></center><br> | ||
| - | Figure 1: The remarkable | + | Figure 1: The remarkable increase of AHL prove the function of immobilized generator cells which secrete AHL continually to the culture flux.<br> |
<center>[[Image:TJU4LI.jpg|500px]]<br><br><br></center> | <center>[[Image:TJU4LI.jpg|500px]]<br><br><br></center> | ||
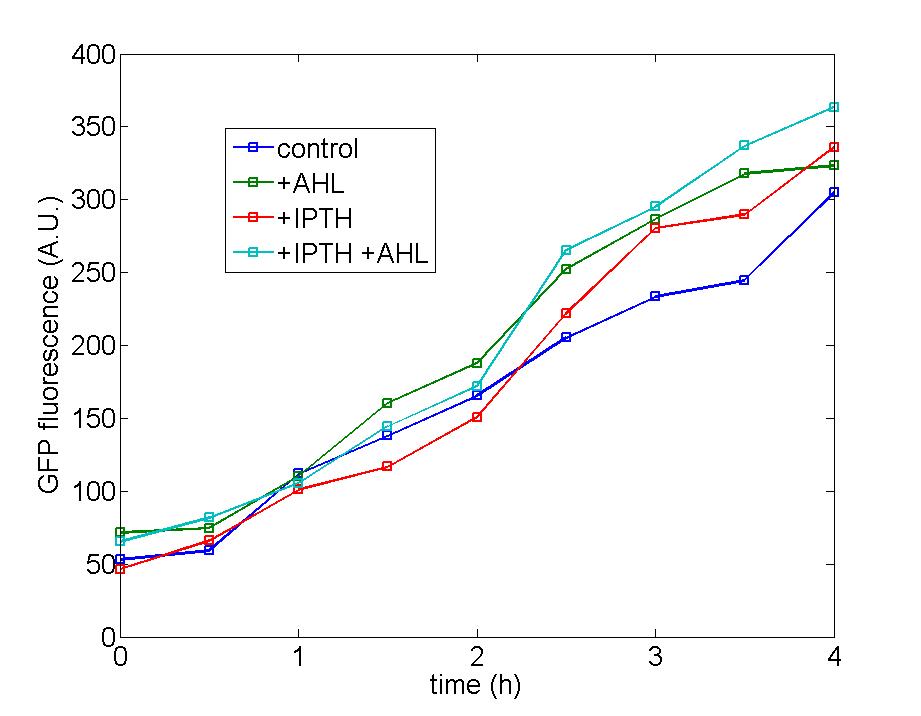
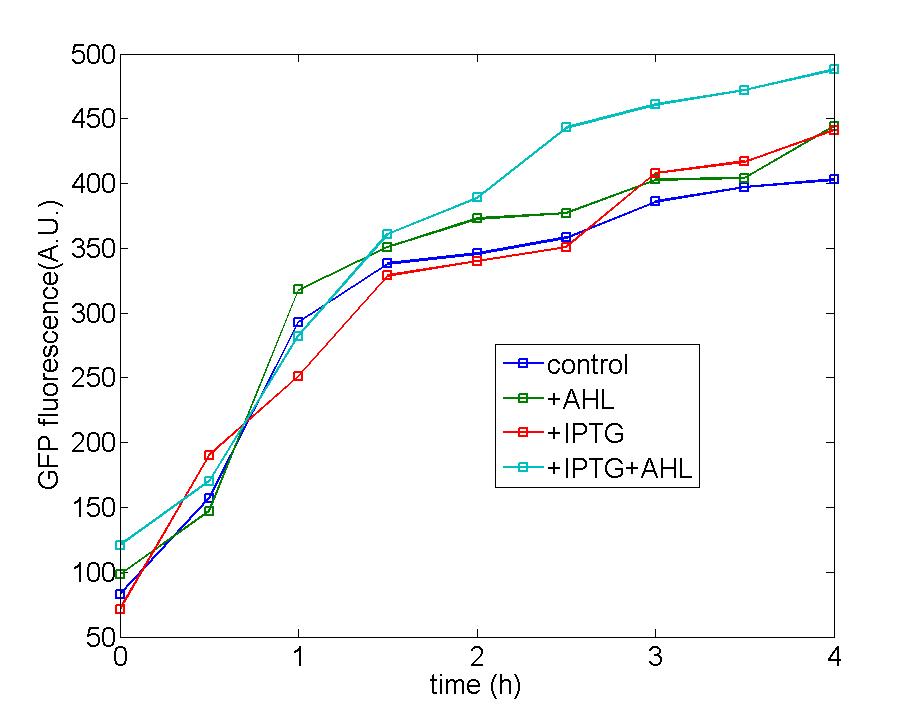
<center><font size="5" color="#0000CC">3.</font><font size="5" color="#0000CC">Function of Amplifier cells</font></center><br> | <center><font size="5" color="#0000CC">3.</font><font size="5" color="#0000CC">Function of Amplifier cells</font></center><br> | ||
| - | Figure 1: Following two figures are selected from five parallel experiments aiming at verifying the function of immobilized amplified cells. The function curves of generator cells and block cells are also displayed in the same figure to emphasize the effects of amplified cells. Actually, the curves of amplified cell( marked as 8P) is identical with that of generator cells, a | + | Figure 1: Following two figures are selected from five parallel experiments aiming at verifying the function of immobilized amplified cells. The function curves of generator cells and block cells are also displayed in the same figure to emphasize the effects of amplified cells. Actually, the curves of amplified cell( marked as 8P) is identical with that of generator cells, a phenomena which could be attribute to self-induction of amplified cells because of its own leakage expression of AHL . Since the threshold of Quorum Sensing is far below than our expectation, further adjustment need to make to eliminate the leakage expression of AHL to a reasonable level to avoid such a condition. |
<br> | <br> | ||
[[Image:TJUQ1.jpg|400px]][[Image:TJUQ2.jpg|400px]] | [[Image:TJUQ1.jpg|400px]][[Image:TJUQ2.jpg|400px]] | ||
<br><br><br><br> | <br><br><br><br> | ||
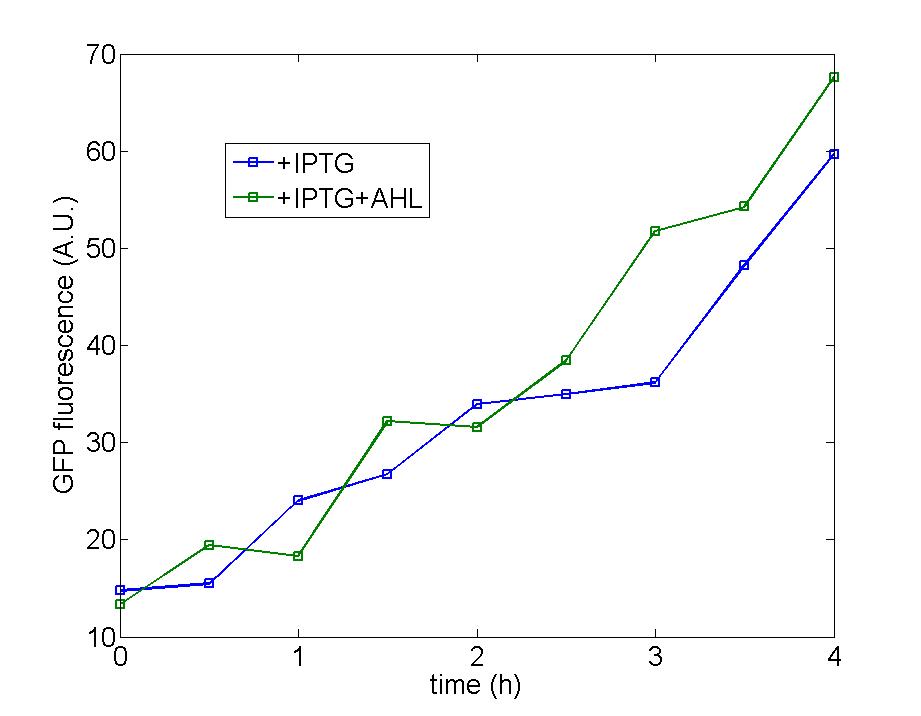
| - | Figure 2: One of our strategy is to cut down the functional part from original plasmid and transfer into a plasmid with a copy number less than 10 without the addition of IPTG (pSB2K3). With the addition of IPTG,the copy number of this plasmid could rise to as much as more than 100, so enhancement of the amplification could be achieved. Although the result is not ideal as that of other types of cells, the graph equally shows AHL signal could be slightly magnified after the addition of IPTG and AHL. The background expression of AHL still stays at a high level which makes the curve of control identical to those added with IPTG and AHL. However, | + | Figure 2: One of our strategy is to cut down the functional part from original plasmid and transfer into a plasmid with a copy number less than 10 without the addition of IPTG (pSB2K3). With the addition of IPTG,the copy number of this plasmid could rise to as much as more than 100, so enhancement of the amplification could be achieved. Although the result is not ideal as that of other types of cells, the graph equally shows AHL signal could be slightly magnified after the addition of IPTG and AHL. The background expression of AHL still stays at a high level which makes the curve of control identical to those added with IPTG and AHL. However, comparing the sapphirine curve with the blue curve, we can see after two hours, the AHL signal is remarkably magnified after the addition of IPTG and AHL. The right graph equally substantiates our hypothesis where the green and blue curve represents the cells with and without induction of IPTG respectively. |
| - | + | <br> | |
| + | Comparing with our [[Tianjin/DIODE/Model2|modeling result]] of amplifier cells, our experiment is not pefect, but the following result show that the amplifier cell can achieve a different AHL concentration when AHL was added immediately the IPTG was added which shows that the amplifier cell can amplify the input AHL signal at a certain extent.<br> | ||
[[Image:TJU501.jpg|300px]] [[Image:TJU502.jpg|300px]] [[Image:TJU503.jpg|300px]] | [[Image:TJU501.jpg|300px]] [[Image:TJU502.jpg|300px]] [[Image:TJU503.jpg|300px]] | ||
<br> | <br> | ||
[[Image:TJU504.jpg|300px]] | [[Image:TJU504.jpg|300px]] | ||
Latest revision as of 01:38, 27 October 2007
Introduction
Because the AHL signal has to be transferred between different kinds of cell which must be separately cultured according to the principles of our design,we decide to immobilize the generator cell(BBa_I13202),amplifier cells(BBa_I15030),block cells(BBa_I13208) using alginate sodium.
Another advantage of immobilizing the amplifier cell is that we want to control the positive feedback which acted as Quorum Sensing in nature by input AHL molecular to cause an input decided system from a time-decided QS system.
After immobilizing these cells ,we test them by samplling the culture medium of the tested cells at different time and mixing it together with detector cell for 1.5h to measure the fluorescence intensity. All the fluorescence intensity is quantificationally measured by Virian Cary Eclipse fluorescence spectrophotometer using the 3ml cup at following condition : Excitation wavelength-501nm(5nm),Emission wavelength-511nm(5nm),Average time 0.5s.
The function of the generator and block cell is the same as what we expect but the function of the amplifier cells is still waiting to be improved. As our experiment discovered, the amplifier cells can produce a lot of AHL because of the induction of leakage expression of AHL by themselves, so the positive feedback permanently exists because there is no difference between the AHL generating rate of the amplifiers with or without AHL added at the beginning.So we speculate that we have immobilized the cells whose expression of LuxI gene is persistent and can not be inhibited because of the abundant AHL in the culture and in the alginate sodium beads which is hard to eliminate. Then we tried to treat these alginate sodium beads with sodium hydroxide in which the AHL will decompose more quickly .We tested it under various conditions but only to find that the addition of sodium hydroxide is toxic to cells and severely damages its metabolic activities ,so we have to abandon this method to improve the performance of amplifier cells.
Then we cut off the function part BBa_I13202 to ligate it with another vector pSB2K3 whose copy number is controlled by IPTG hoping that after induction of IPTG simultaneously with AHL could boost the duplication of functional part and thus to produce far more AHL than self-induced cells. The experimental results indicate that the performance of amplifier cells has been ameliorated a little bit.
Test result
All the result of fluorescence values is based on the function of our detector cell which is shown here.
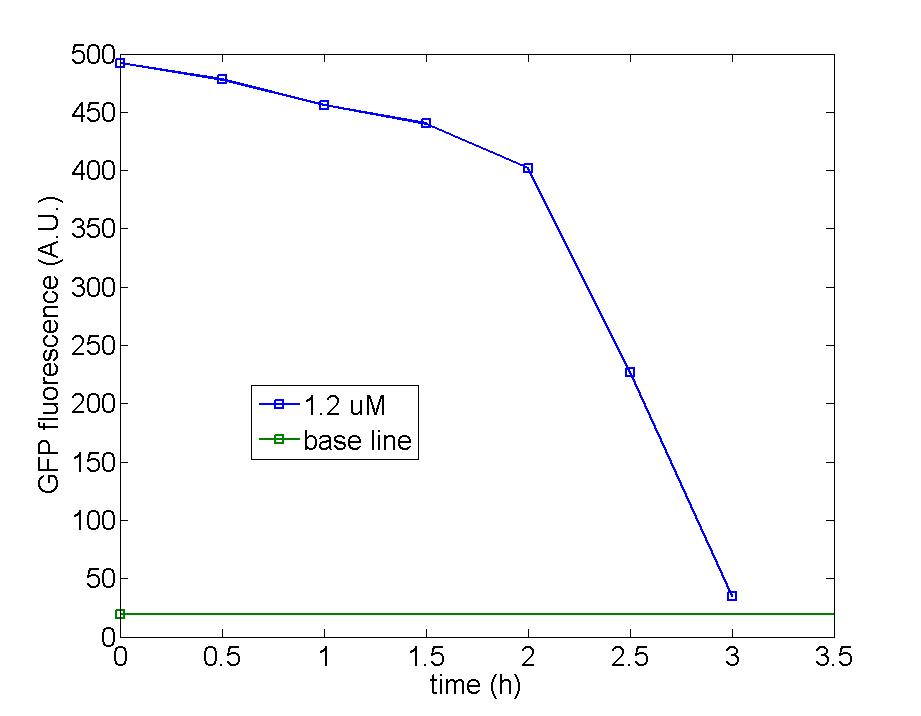
Figure 1: AHL tends to be degraded gradually by immobilized block cells as both figure indicated, after 7 hours, the concentration of AHL has dropped down to less than half of the original value. The difference between the time needed to observe remarkable decline is because the number of immobilized beads on the left figure outnumbered that on the right. Therefore, block cells still work well after being immobilized.
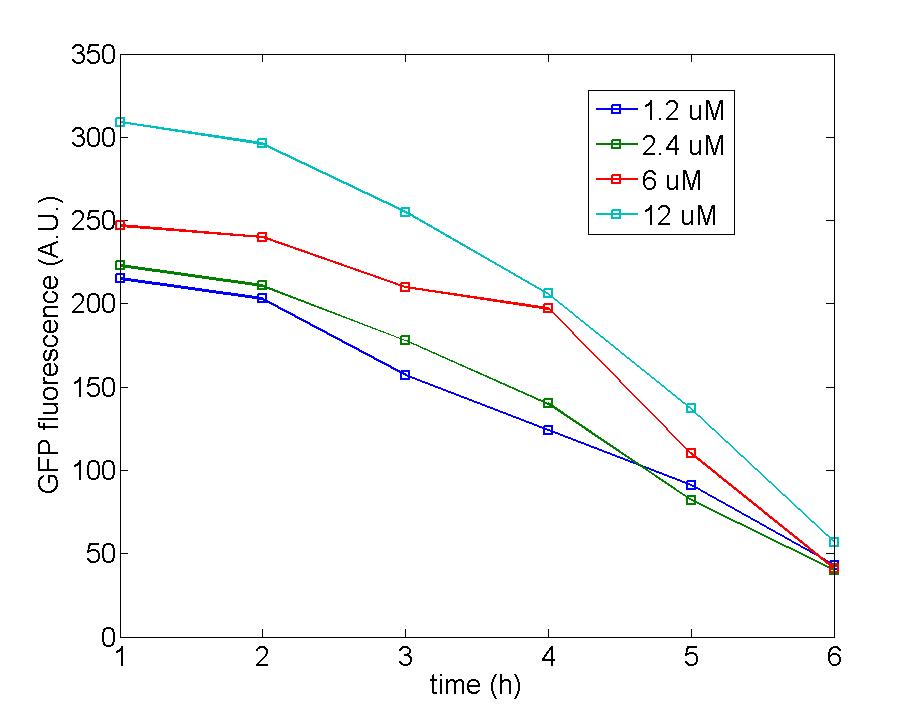
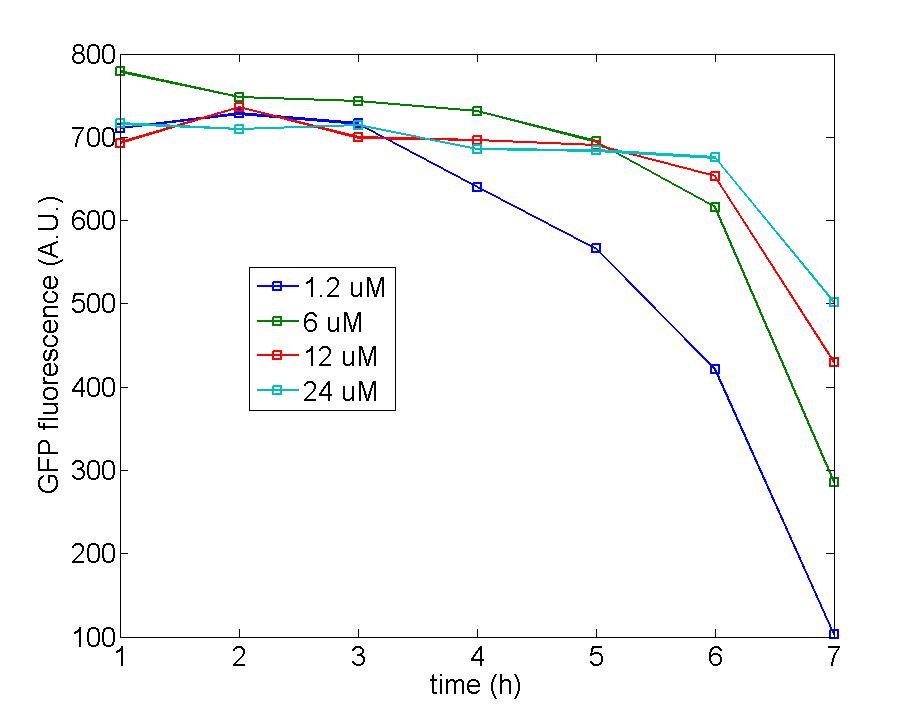
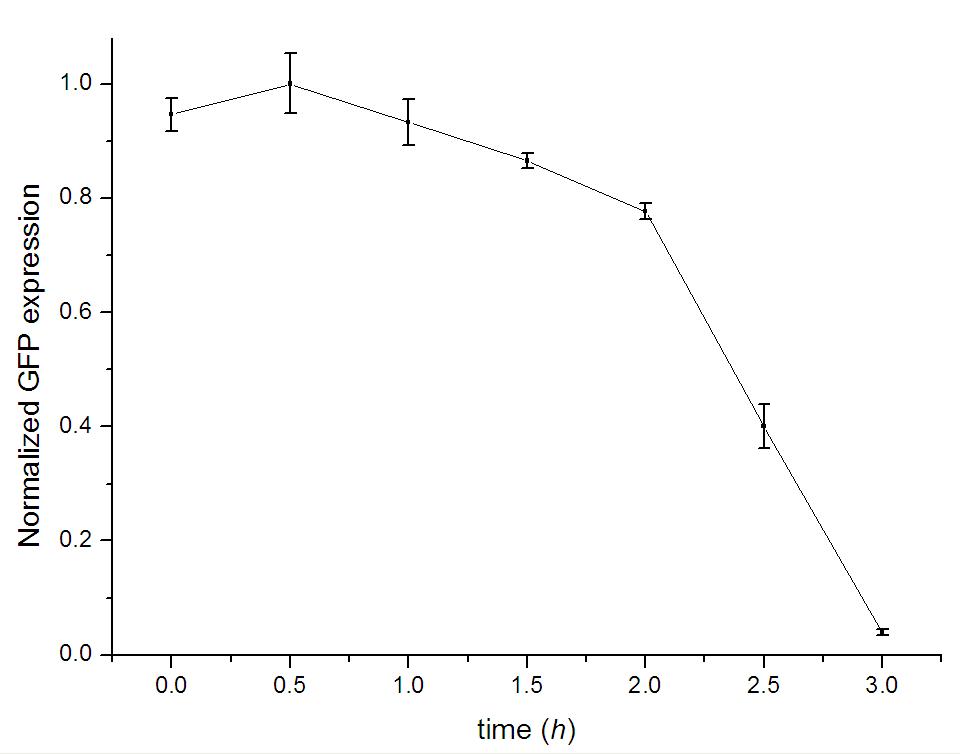
Figure 2: To test the validity of our data, the background expression of detector cells and standard deviation of our data are also considered. As the figures show, the leakage expression of GFP is far below the level of activated expression and influent a little on final curves and the tendency of curves would not change within the range of standard deviation.
Figure 1: The remarkable increase of AHL prove the function of immobilized generator cells which secrete AHL continually to the culture flux.
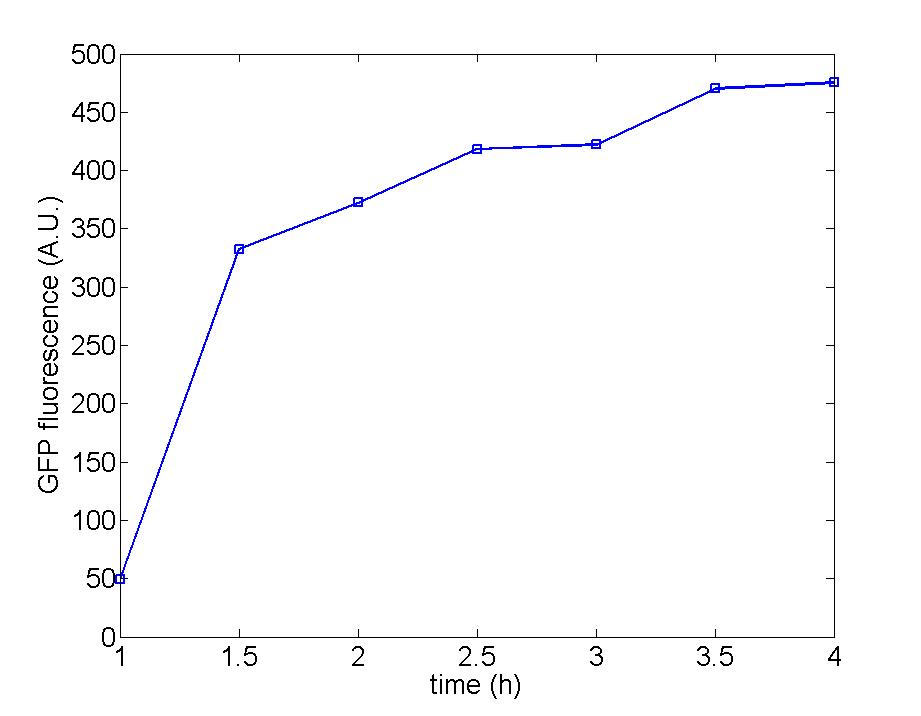
Figure 1: Following two figures are selected from five parallel experiments aiming at verifying the function of immobilized amplified cells. The function curves of generator cells and block cells are also displayed in the same figure to emphasize the effects of amplified cells. Actually, the curves of amplified cell( marked as 8P) is identical with that of generator cells, a phenomena which could be attribute to self-induction of amplified cells because of its own leakage expression of AHL . Since the threshold of Quorum Sensing is far below than our expectation, further adjustment need to make to eliminate the leakage expression of AHL to a reasonable level to avoid such a condition.
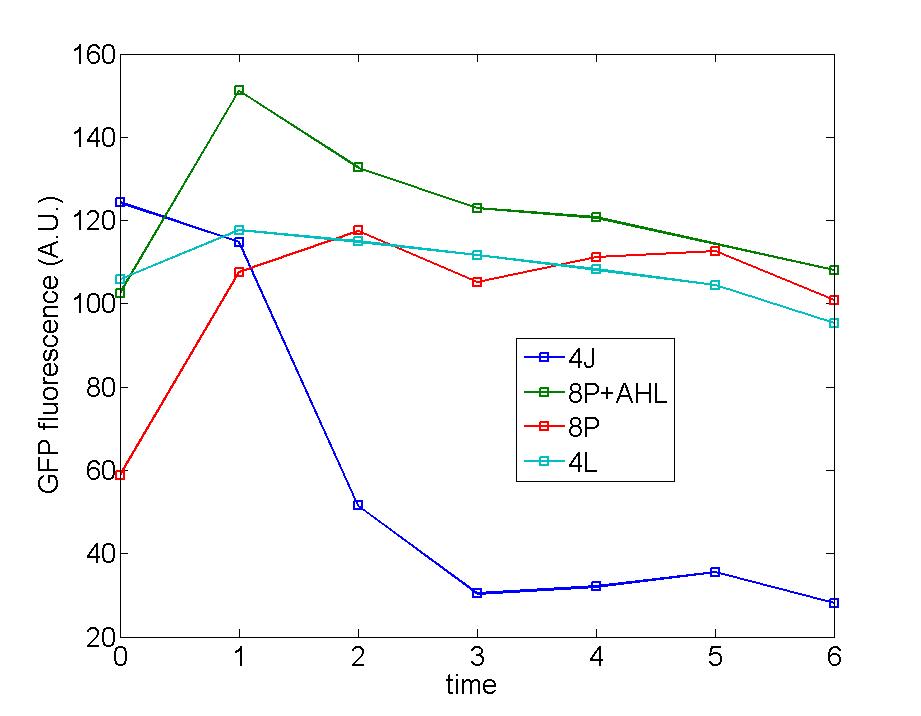
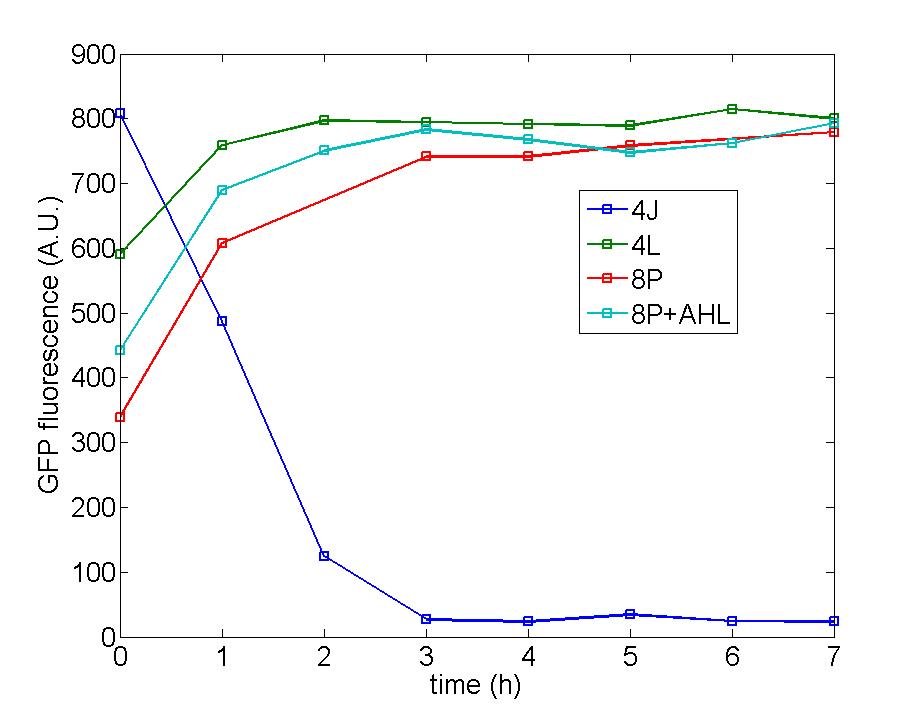
Figure 2: One of our strategy is to cut down the functional part from original plasmid and transfer into a plasmid with a copy number less than 10 without the addition of IPTG (pSB2K3). With the addition of IPTG,the copy number of this plasmid could rise to as much as more than 100, so enhancement of the amplification could be achieved. Although the result is not ideal as that of other types of cells, the graph equally shows AHL signal could be slightly magnified after the addition of IPTG and AHL. The background expression of AHL still stays at a high level which makes the curve of control identical to those added with IPTG and AHL. However, comparing the sapphirine curve with the blue curve, we can see after two hours, the AHL signal is remarkably magnified after the addition of IPTG and AHL. The right graph equally substantiates our hypothesis where the green and blue curve represents the cells with and without induction of IPTG respectively.
Comparing with our modeling result of amplifier cells, our experiment is not pefect, but the following result show that the amplifier cell can achieve a different AHL concentration when AHL was added immediately the IPTG was added which shows that the amplifier cell can amplify the input AHL signal at a certain extent.