McGill/Results
From 2007.igem.org
(→Reproducing Last Year's System) |
|||
| (21 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
This year our new team has been developing the project established last year which you can see on the McGill 2006 wiki. We have made many new discoveries and have run into some problems hindering our progress along the way; nevertheless, we were able to make some new developments as outlined below: | This year our new team has been developing the project established last year which you can see on the McGill 2006 wiki. We have made many new discoveries and have run into some problems hindering our progress along the way; nevertheless, we were able to make some new developments as outlined below: | ||
| - | + | ||
| - | The primary oscillating system, the I15004 with the J40001 as outlined in the previous pages was produced last year and viewed using a microscope for several hours to analyze the oscillatory period and confirm the presence of these oscillations. | + | == Reproducing Last Year's System == |
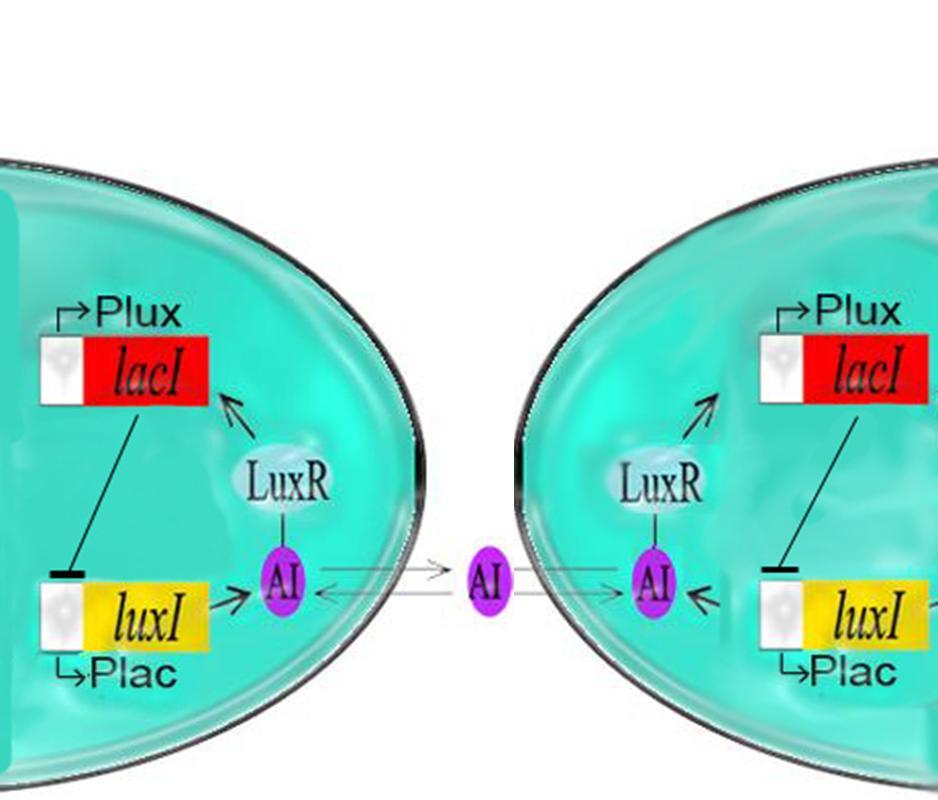
| - | + | [[Image:2gene oscillator.jpg||thumb|left|200px|Two cells displaying the Oscillator]]The primary oscillating system, the I15004 with the J40001 as outlined in the previous pages was produced last year and viewed using a microscope for several hours to analyze the oscillatory period and confirm the presence of these oscillations. [[McGill/Results/Method_1|Click here for methods]]<br> | |
| - | + | From these experiments, we were able to come up with some interesting results. | |
| - | + | ||
| - | + | 1. The more concentrated the cells, the worse the apparent oscillations.<br> | |
| - | + | 2. The presence of Dox seemed to stabilize the oscillations.<br> | |
| - | + | 3. The greater the amount of AHL, the worse the apparent oscillations, as predicted with the simulations.<br> | |
| - | ''' | + | 4. Using an optical density assay, it was found that the cells were in fact dying (or clumping) thus lowering the optical density throughout the testing period.<br> |
| - | + | 5. Oscillations occured with the J40001 (pLux-LacI-ECFP) alone. The reason for this could be some interaction with the expression of endogenous Lac in the BL21 cell line we used. | |
| + | <br> | ||
| + | We replaced the BL21 cells with MC4100 cells, the lac-, tet- cells used by Elowitz himself. <br> | ||
| + | '''Results:''' no oscillations which led us to the next point, the malfunctioning I15004 brick needs to be replaced. | ||
| + | ---- | ||
| + | <br> | ||
| + | |||
| + | == Production of a new I15004 biobrick == | ||
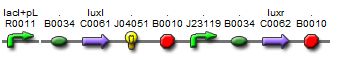
| + | [[Image:newI.jpg||thumb|left|400px|Sequence of the new I15004 biobrick]]In light of the terrible bands following a restriction digest and the lack of oscillations in the MC cells, we constructed a new sequence of DNA containing the proper components of the I15004 needed for the oscillating network and added of few of our own.<br><br><br><br> | ||
| + | |||
| + | This sequence contains the following:<br> | ||
| + | 1. pLac, ribosome binding site, LuxI These three components are present from the I15004 and are required for the oscillating network.<br> | ||
| + | 2. RFP The RFP is our own addition. It allows us to track the expression of both the oscillating I15004 brick and the J40001 brick allowing us to evaluate oscillations in more than one way (note, a third method of reporting is included with the repressillator).<br> | ||
| + | 3. pConstitutive, ribosome binding site, LuxR Inculsion of this constitutive promoter was another one of our design ideas. When coupled with the repressilator, this eliminates one of the crossing links that would make the system more connected and create more variables. By eliminating the tetracycline promotor, our system is much cleaner.<br> | ||
| + | |||
| + | '''As ideal and wonderful as this system sounds, we were unable to put it into action. We ordered the sequence from GenArt in early August and it has still not arrived in time for the Jamboree due to ligation problems at the company.''' | ||
| + | |||
| + | ---- | ||
| + | |||
| + | <br> | ||
| + | == Coupling of the Oscillator with the Repressilator == | ||
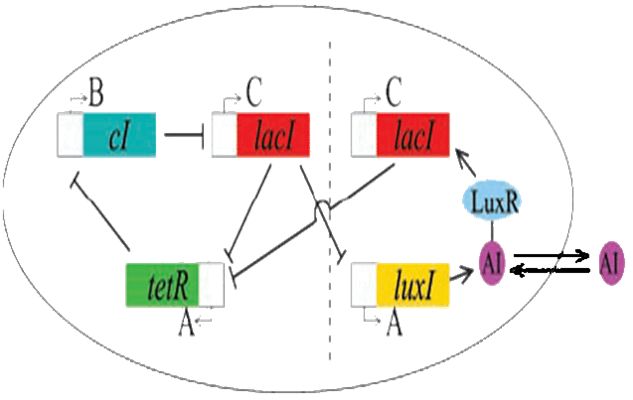
| + | [[Image:osc-rep.jpg||thumb|left|300px|The Oscillator coupled with the Elowitz Repressilator]]As per the future prospects presented in 2006, one of the major goals of this year was to couple the oscillator with Elowitz's repressilator: a triple repressing system which will change the spiking oscillations of the dual oscillator to robust sinosodial curves. | ||
| + | <br> Modelling using deterministic rate equations predicted beautiful curves that we have attempted to reproduce in the lab. Again, we ran into some problems. Repeated restriction digests produced anomalous gel results and when attempting to view the reporting gene in the repressilator, we were unable to see the fluorescence. | ||
| + | <br>Our conclusion is that the I5610 is not what is appears to be. So, onto our next section : creating a new represillator. Thus, coupling was not yet possible in practice. | ||
| + | |||
| + | |||
| + | ---- | ||
| + | |||
| + | <br> | ||
| + | == Synthesizing a new Repressilator == | ||
| + | [[Image:new_rep.jpg||thumb|left|500px|The new and improved I5611 Repressilator brick!]]In the registry, there are two alternative biobricks that contain fragments of the repressilator. One of them, I5611, contains all the components of the represillator without the degradation tag for the flourescent reporter.<br> | ||
| + | We placed a new degradation tag on the I5611 to make a functional Elowitz repressilator. Another biobrick, E0434, contains the same YFP reporter with a functional degradation tag. Using BsrG1 and SpeI to cleave the YFP at an identical point in both the I5611 and the E0434, we plan to preform a ligation of these two pieces to develop the functional unit.<br><br> | ||
| + | Unfortunately, though in progress, this could not be completed for the Jamboree due to ligation difficulty. Due to the low copy number of the plasmids to be amplified and the fidelity of the ligation proceedure, time did not permit the completion of this vital component of our project thus there are no represillator oscillations to report. | ||
| + | |||
| + | ---- | ||
| + | |||
| + | <br> | ||
| + | == Synthesizing an AI (AHL) Inhibitor into our J40001 biobrick == | ||
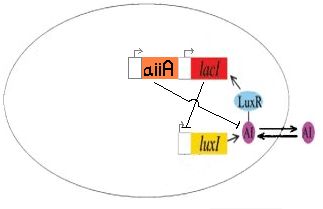
| + | [[Image:aiiAsystem.jpg||thumb|left|250px|The AI Inhibitor (aiiA) shown here ligated to the J40001 biobrick, used to control AI degradation]]We completed the construction of I724000, the autoinducer inactivation enzyme regulated by LuxR/HSL promoter, by ligating R0062, B0034 and C0060. This part has been submitted to the registry as a functional version of the aiiA gene. <br> | ||
| + | We are currently still working on incorporating this construct into J40001 to create I724001 (LacI + ECFP under luxpR, with autoinducer inactivation). | ||
| + | <br><br>Sadly we were unable to complete the ligation in time to submit the part to the registry by the deadline. | ||
Latest revision as of 02:46, 27 October 2007
This year our new team has been developing the project established last year which you can see on the McGill 2006 wiki. We have made many new discoveries and have run into some problems hindering our progress along the way; nevertheless, we were able to make some new developments as outlined below:
Contents |
Reproducing Last Year's System
The primary oscillating system, the I15004 with the J40001 as outlined in the previous pages was produced last year and viewed using a microscope for several hours to analyze the oscillatory period and confirm the presence of these oscillations. Click here for methodsFrom these experiments, we were able to come up with some interesting results.
1. The more concentrated the cells, the worse the apparent oscillations.
2. The presence of Dox seemed to stabilize the oscillations.
3. The greater the amount of AHL, the worse the apparent oscillations, as predicted with the simulations.
4. Using an optical density assay, it was found that the cells were in fact dying (or clumping) thus lowering the optical density throughout the testing period.
5. Oscillations occured with the J40001 (pLux-LacI-ECFP) alone. The reason for this could be some interaction with the expression of endogenous Lac in the BL21 cell line we used.
We replaced the BL21 cells with MC4100 cells, the lac-, tet- cells used by Elowitz himself.
Results: no oscillations which led us to the next point, the malfunctioning I15004 brick needs to be replaced.
Production of a new I15004 biobrick
In light of the terrible bands following a restriction digest and the lack of oscillations in the MC cells, we constructed a new sequence of DNA containing the proper components of the I15004 needed for the oscillating network and added of few of our own.This sequence contains the following:
1. pLac, ribosome binding site, LuxI These three components are present from the I15004 and are required for the oscillating network.
2. RFP The RFP is our own addition. It allows us to track the expression of both the oscillating I15004 brick and the J40001 brick allowing us to evaluate oscillations in more than one way (note, a third method of reporting is included with the repressillator).
3. pConstitutive, ribosome binding site, LuxR Inculsion of this constitutive promoter was another one of our design ideas. When coupled with the repressilator, this eliminates one of the crossing links that would make the system more connected and create more variables. By eliminating the tetracycline promotor, our system is much cleaner.
As ideal and wonderful as this system sounds, we were unable to put it into action. We ordered the sequence from GenArt in early August and it has still not arrived in time for the Jamboree due to ligation problems at the company.
Coupling of the Oscillator with the Repressilator
As per the future prospects presented in 2006, one of the major goals of this year was to couple the oscillator with Elowitz's repressilator: a triple repressing system which will change the spiking oscillations of the dual oscillator to robust sinosodial curves.
Modelling using deterministic rate equations predicted beautiful curves that we have attempted to reproduce in the lab. Again, we ran into some problems. Repeated restriction digests produced anomalous gel results and when attempting to view the reporting gene in the repressilator, we were unable to see the fluorescence.
Our conclusion is that the I5610 is not what is appears to be. So, onto our next section : creating a new represillator. Thus, coupling was not yet possible in practice.
Synthesizing a new Repressilator
In the registry, there are two alternative biobricks that contain fragments of the repressilator. One of them, I5611, contains all the components of the represillator without the degradation tag for the flourescent reporter.We placed a new degradation tag on the I5611 to make a functional Elowitz repressilator. Another biobrick, E0434, contains the same YFP reporter with a functional degradation tag. Using BsrG1 and SpeI to cleave the YFP at an identical point in both the I5611 and the E0434, we plan to preform a ligation of these two pieces to develop the functional unit.
Unfortunately, though in progress, this could not be completed for the Jamboree due to ligation difficulty. Due to the low copy number of the plasmids to be amplified and the fidelity of the ligation proceedure, time did not permit the completion of this vital component of our project thus there are no represillator oscillations to report.
Synthesizing an AI (AHL) Inhibitor into our J40001 biobrick
We completed the construction of I724000, the autoinducer inactivation enzyme regulated by LuxR/HSL promoter, by ligating R0062, B0034 and C0060. This part has been submitted to the registry as a functional version of the aiiA gene.We are currently still working on incorporating this construct into J40001 to create I724001 (LacI + ECFP under luxpR, with autoinducer inactivation).
Sadly we were unable to complete the ligation in time to submit the part to the registry by the deadline.