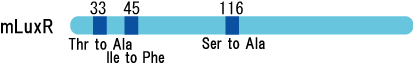Chiba/Quorum Sensing
From 2007.igem.org
(→Design) |
(→Experiment) |
||
| (151 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
[[Image:chiba_logo.png|center]] | [[Image:chiba_logo.png|center]] | ||
__NOTOC__ | __NOTOC__ | ||
| - | {| style="border:0;width:100%;" cellpadding="20px" cellspacing="0" | + | {| style="border:0;width:100%;font-family:'Trebuchet MS'" cellpadding="20px" cellspacing="0" |
| align="center" | | | align="center" | | ||
| - | [[Chiba|Introduction]] | [[Chiba/Project_Design|Project Design]] ( [[Chiba/Engeneering_Flagella|1. | + | [[Chiba|Home]]<br> |
| + | <span style="font-size:120%;font-weight:bold;">[[Chiba/Introduction|Introduction]] | [[Chiba/Project_Design|Project Design]] ( [[Chiba/Engeneering_Flagella|1.Affinity Tag]] | [[Chiba/Communication|2.Communication Module]] | [[Chiba/Quorum_Sensing|3.Size Control]] ) | [[Chiba/Making Marimo|Making Marimos]] | [[Chiba/Goal|Our Goal]]</span><br> | ||
| + | [[Chiba/Acknowledgements|Acknowledgements]] | [[Chiba/Team_Members|Team Members]] | [http://chem.tf.chiba-u.jp/igem/ iGEM Chiba Website] | [[Chiba/Members_Only|メンバ連絡簿]] | ||
|} | |} | ||
| - | ==Size Control | + | ==Size Control== |
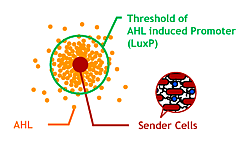
| - | [[Image:Chiba_proj_ahlconc.png|frame|''' | + | ===Our Aim=== |
| - | + | [[Image:Chiba_proj_ahlconc.png|frame|'''Fig. 15''' Controlling AHL diffusing area and the size of Bacteria Marimo.]] | |
| - | + | Since produced AHL diffuses all around the bacteria culture, all receivers can ultimately aggregate to one huge marimo with their sticky hands. This final state is not the case of real marimos. Thus our idea for controlling the size of Bacteria Marimos is based on the high performance quorum sensing or AHL-diffusing inhibition. | |
| - | + | #Raise the AHL productivity of Sender | |
| - | + | #Increase the AHL sensitivity of Receiver | |
| - | + | #Use the AHL degrading enzyme aiiA to localize AHL | |
<br clear="all"> | <br clear="all"> | ||
| Line 21: | Line 23: | ||
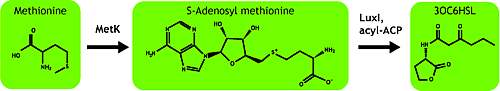
AHL is synthesized from methionine by the enzyme MetK and LuxI.<br> | AHL is synthesized from methionine by the enzyme MetK and LuxI.<br> | ||
| - | We | + | We tested in the hope of combined overexpression of these 2 genes will enhance the sender capacity. |
| - | + | <br>[[Image:Chiba_ahlsynth.png|frame|'''Fig. 16''' Passway synthesizing AHL in bacteria.]] | |
| - | <br>[[Image:Chiba_ahlsynth.png|frame|Fig. | + | |
<br clear="all"> | <br clear="all"> | ||
===Experiment=== | ===Experiment=== | ||
| - | [[Image:Chiba AHL test 02.jpg]] | + | [[Image:Chiba AHL test 02.jpg|frame|'''Fig. 17''' Testing procedure of sender capability.]] |
====Sender==== | ====Sender==== | ||
| - | + | Ptet-LuxI | |
| - | + | ||
*Synthesize AHL constantly | *Synthesize AHL constantly | ||
====metK Sender==== | ====metK Sender==== | ||
[[Image:metK circuit.jpg]] | [[Image:metK circuit.jpg]] | ||
| - | *Synthesize AHL | + | *Synthesize AHL / express metK (precursor supplier) constantly |
====Receiver==== | ====Receiver==== | ||
| - | [[Image:BBa T9002.jpg]] | + | [[Image:BBa T9002.jpg|350x48px]] |
| + | *BBa T9002 (Express GFP in response to AHL) | ||
| + | |||
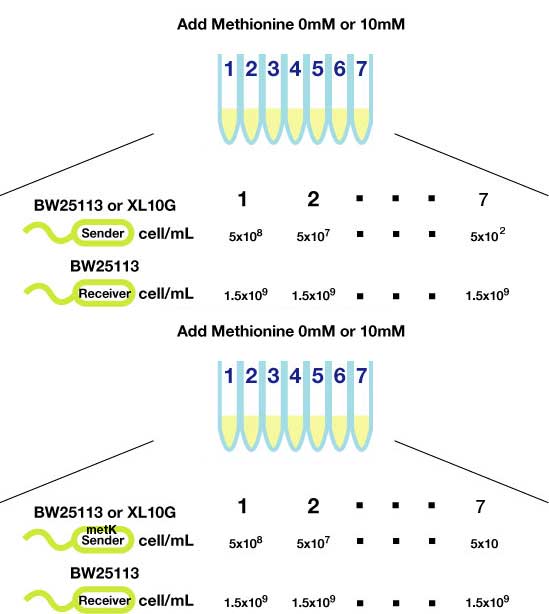
| + | ====Method(Fig3.)==== | ||
| + | #Transform sender (Ptet-luxI) and MetK sender (pLac-luxI-metK) into E coli strains (XL10Gold and BW25113) | ||
| + | #Inoculated them independently in liquid media. Incubated at 37℃ 12h. | ||
| + | #Checked OD<sub>600</sub>, washed, and diluted senders to adjust cell number (5x10<sup>8</sup>,5x10<sup>7</sup>,......, 5x10<sup>2</sup>). | ||
| + | #Mix with receiver (pLux-GFP/ BW25113, 1.5x10<sup>9</sup> cells) | ||
| + | #Shake for 1-3h at room tempature (optonal; methionine (10mM) added) | ||
| + | #Spindowned and fluorescence checked. | ||
===Result=== | ===Result=== | ||
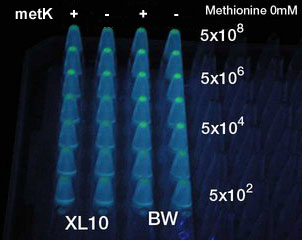
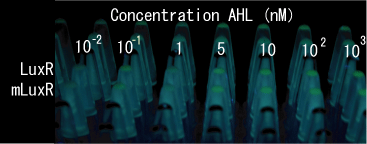
| - | + | [[Image:AHL test photo 01.jpg|frame|left|'''Fig. 18''' Testing minimum amount of senders required for activating receivers.<br> ]] | |
| - | [[Image:AHL test photo 01.jpg| | + | <br clear="all"> |
| - | + | *At least 5x10<sup>4</sup> cell/ml sender required to activate lux promoter in BW25113. | |
| + | *Sender performance HIGHLY depends on the strains: 100x more cells were required to activate the lux promoter in other strain XL10Gold. | ||
| + | *Overexpression of metK did not result in the booster effect. It was so even when Methionine (10mM), the direct substrate of metK, was added to the growth media (not shown). Sucks. | ||
===Discussion=== | ===Discussion=== | ||
| - | * | + | *No improbement in sending power was observed. |
| + | *Assumption (not conclusion):<br> | ||
| + | (1) Precursor supply (S-adenosyl methionine) is simply at enough level, and luxR is by far the rate-limiting step in AHL synthetic pathway. No chance/ need for metabolic engineering of this pathway. <br> | ||
| + | (2) Precursor flows out to somewhere else. Or overexpression of metK ativates the degradation pathway of its product. Anyway too hard for us to cope with in short time. | ||
| + | <br> | ||
| + | *Note; We haven't confirmed the entire sequencing of the metK. | ||
| - | + | ==2.Sensitizing Receiver== | |
| - | ==2. | + | |
===Design=== | ===Design=== | ||
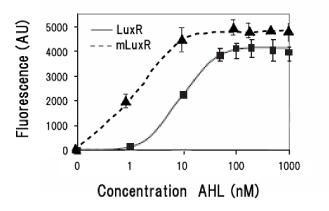
| - | [[Image: | + | [[Image:MLuxRx.gif|frame|left|'''Fig. 19''' Transfer curve of LuxR mutant to AHL.''' Data modified from Collins ''et.al.'' Mol. Microbiol. 55, 712–723 (2005)''']][[Image:MLuxRxxx.gif|frame|left|'''Fig. 20''' LuxR mutations focused in our project ]]<br clear="all"> |
| - | Collins et al described the hyper-sensitive variants of luxR to AHL | + | Collins ''et.al.'' described the hyper-sensitive variants of luxR to AHL.(Collins, C. H., Arnold, F. H. & Leadbetter, J. R. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. ''Mol. Microbiol.'' '''55''', 712–723 (2005))<br> |
| - | We created two | + | We created two luxR mutants. One is I14F mutant of LuxR, and another is I45F/S116A.<br> |
| + | There is no report on I45F/S116A double mutant.By creating/ analyzing this mutant in AHL sensitivity, we can tell the effect of T33A in LuxR.<br> | ||
===Experiment=== | ===Experiment=== | ||
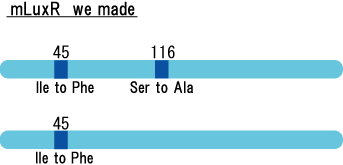
| - | [[Image: | + | [[Image:MLuxRxx.gif|frame|'''Fig. 21''' Location of the mutations in luxR gene]]<br> |
| - | + | luxR mutants was analyzed in their sensitivity to AHL by following procedure.<br> | |
| + | |||
| + | #Inoculate Receiver (wild type luxR/BW25113), mutated Receiver (1point mutation/BW25113) in liquid media for 12 h at 37℃. | ||
| + | #Adjust the cell density and add AHL (final concentration, 0, 0.1, 1, 5, 10, 100, and 1000 nM) | ||
| + | #Incubate for 1h at room temperature | ||
| + | #Spindown cells and GFP folurescent level checked<br><br><br> | ||
===Result=== | ===Result=== | ||
| - | *mutant(I45F,S116A) | + | [[Image:MLuxR-test.gif|frame|left|'''Fig. 22''' comparison 1point mutated and wild type]] |
| - | [[Image: | + | *Both wild type and 1point mutation luxR express GFP under 5nM AHL concentration. |
| + | <br><br><br><br><br><br><br><br><br> | ||
| + | [[Image:Two mutation.jpg|320x240px|frame|left|'''Fig. 23''' LuxR mutant with two point mutations expressed GFP without adding AHL]] | ||
| + | *2point mutation(I45F,S116A) luxR Receiver express GFP without AHL. | ||
| + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
| + | |||
| + | ===Discussion=== | ||
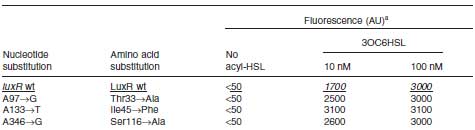
| + | [[Image:MluxR_table.jpg |frame|left|'''Table 1 Seisitivity of LuxR mutants.''' Data modified from Collins ''et.al.'' Mol. Microbiol. 55, 712–723 (2005)]]<br><br><br><br><br><br><br><br><br> | ||
| - | |||
| + | This table is quoted from paper.<br> | ||
| + | In the paper, DH5α strain was used.<br> | ||
| + | But wild type luxR in our study expressed GFP under 5nM AHL concentration.<br> | ||
| - | + | Assumption:There is some difference between strains. | |
| + | Required to do same test on other strains | ||
| - | ==3. | + | ==3.Localizing AHL== |
| - | + | ||
| - | + | ||
| - | + | ||
===Design1=== | ===Design1=== | ||
| - | [[Image:BBa I729006 circuit.jpg]] | + | [[Image:BBa I729006 circuit.jpg]]<Br> |
| - | AiiA | + | BBa I729006<br> |
| + | In addition to the receiver unit, there added AiiA under the control of lac promoter.<br> | ||
| + | AiiA degrades/ inactivates AHL, lowering the AHL concentration in the cell. <br> | ||
| + | This way cells installed with this circuit can respond, but less sensitive, to the sender cells. | ||
====Experiment==== | ====Experiment==== | ||
| - | + | #Inoculate E.coli carries this aiiA receiver plasmid in Liquid Media. | |
| - | + | #At the same time, inoculate AHL sender E.coli in Liquid Media. | |
| - | + | #Dilute receiver cells and spread on agar plate, and spot sender 1μL. Incubate at 37℃ 12hour | |
| - | + | #Check plate.(GFP expressed?) | |
| + | |||
====Result==== | ====Result==== | ||
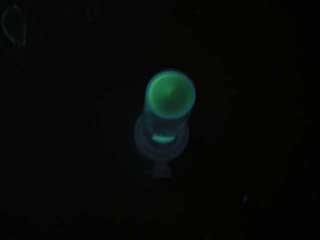
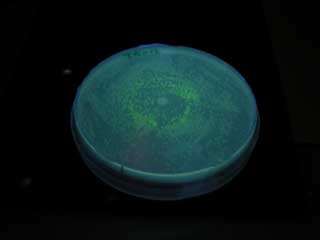
| - | GFP | + | GFP expression was not observed on the plate of aiiA receiver. |
| - | [[ | + | |
| + | [[Image:T9002 plate.jpg|frame|'''Fig. 24''' Receiver|left]] | ||
| + | [[Image:AiiA cons plate.jpg|frame|'''Fig. 25''' aiiA receiver|center]] | ||
| + | <br clear="all"> | ||
| + | |||
====Discussion==== | ====Discussion==== | ||
| - | + | We thought expression of aiiA significantly decrease the cellular level of AHL. It turned out to be true.<br> | |
| - | We | + | However, it went too much. It does not respond to senders anymore, probably due to too fast AHL degradation. <br> |
| + | We need more controlled/ moderate expression of aiiA. | ||
===Design2=== | ===Design2=== | ||
[[Image:Plux aiiA.jpg|402x45px]]<br> | [[Image:Plux aiiA.jpg|402x45px]]<br> | ||
| - | + | This gene circuit is expected so that aiiA is synthesized only when the receiver senses AHL signal. If so, the whole Bacteria Marimo becomes AHL quencher. In fact, the bacteria inside of the Marimo degrades AHL and the AHL diffusion outside of Marimo is inhibited. | |
====Experiment==== | ====Experiment==== | ||
| + | #Inoculate E.coli carries this aiiA receiver plasmid in Liquid Media. | ||
| + | #At the same time, inoculate AHL sender E.coli in Liquid Media. | ||
| + | #Dilute receiver cells and spread on agar plate, and spot sender 1μL. Incubate at 37℃ 12hour | ||
| + | #Put plate 30℃ | ||
| + | #Check GFP expression every 1hour. | ||
====Result==== | ====Result==== | ||
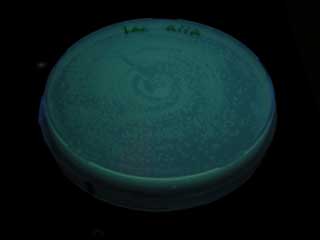
| - | * | + | *When we compared the GFP expression with the test tube of BBa_T9002, the bacteria with the consructed gene circuit did not fluoresce immediately after incubation, however fluoresced in 18~20 hour.<br> |
| - | + | ||
====Discussion==== | ====Discussion==== | ||
| - | * | + | *GFP expression means plux is activated. So we could make moderate aiiA receiver. |
| + | *But we need more twisted circuit. | ||
===Design3=== | ===Design3=== | ||
[[Image:Rec inv aiia.jpg|402x45px]]<br> | [[Image:Rec inv aiia.jpg|402x45px]]<br> | ||
| - | + | Above picture describes aiiA is regulated inverted lux promoter with CI inverter. | |
| + | *High AHL concentration: no aiiA expression, no AHL degrade. | ||
| + | *Low AHL concentration: aiiA expressed, AHL degraded. | ||
| + | |||
| + | |||
| + | |||
====Experiment==== | ====Experiment==== | ||
| - | + | We could not finish assembling this part. It will be our future work. | |
Subpart:BBa_S03840 | Subpart:BBa_S03840 | ||
[[Image:S03840.jpg]] | [[Image:S03840.jpg]] | ||
Latest revision as of 05:41, 27 October 2007
|
Home |
Size Control
Our Aim
Since produced AHL diffuses all around the bacteria culture, all receivers can ultimately aggregate to one huge marimo with their sticky hands. This final state is not the case of real marimos. Thus our idea for controlling the size of Bacteria Marimos is based on the high performance quorum sensing or AHL-diffusing inhibition.
- Raise the AHL productivity of Sender
- Increase the AHL sensitivity of Receiver
- Use the AHL degrading enzyme aiiA to localize AHL
1.Improving Sender
Design
AHL is synthesized from methionine by the enzyme MetK and LuxI.
We tested in the hope of combined overexpression of these 2 genes will enhance the sender capacity.
Experiment
Sender
Ptet-LuxI
- Synthesize AHL constantly
metK Sender
- Synthesize AHL / express metK (precursor supplier) constantly
Receiver
- BBa T9002 (Express GFP in response to AHL)
Method(Fig3.)
- Transform sender (Ptet-luxI) and MetK sender (pLac-luxI-metK) into E coli strains (XL10Gold and BW25113)
- Inoculated them independently in liquid media. Incubated at 37℃ 12h.
- Checked OD600, washed, and diluted senders to adjust cell number (5x108,5x107,......, 5x102).
- Mix with receiver (pLux-GFP/ BW25113, 1.5x109 cells)
- Shake for 1-3h at room tempature (optonal; methionine (10mM) added)
- Spindowned and fluorescence checked.
Result
- At least 5x104 cell/ml sender required to activate lux promoter in BW25113.
- Sender performance HIGHLY depends on the strains: 100x more cells were required to activate the lux promoter in other strain XL10Gold.
- Overexpression of metK did not result in the booster effect. It was so even when Methionine (10mM), the direct substrate of metK, was added to the growth media (not shown). Sucks.
Discussion
- No improbement in sending power was observed.
- Assumption (not conclusion):
(1) Precursor supply (S-adenosyl methionine) is simply at enough level, and luxR is by far the rate-limiting step in AHL synthetic pathway. No chance/ need for metabolic engineering of this pathway.
(2) Precursor flows out to somewhere else. Or overexpression of metK ativates the degradation pathway of its product. Anyway too hard for us to cope with in short time.
- Note; We haven't confirmed the entire sequencing of the metK.
2.Sensitizing Receiver
Design
Collins et.al. described the hyper-sensitive variants of luxR to AHL.(Collins, C. H., Arnold, F. H. & Leadbetter, J. R. Directed evolution of Vibrio fischeri LuxR for increased sensitivity to a broad spectrum of acyl-homoserine lactones. Mol. Microbiol. 55, 712–723 (2005))
We created two luxR mutants. One is I14F mutant of LuxR, and another is I45F/S116A.
There is no report on I45F/S116A double mutant.By creating/ analyzing this mutant in AHL sensitivity, we can tell the effect of T33A in LuxR.
Experiment
luxR mutants was analyzed in their sensitivity to AHL by following procedure.
- Inoculate Receiver (wild type luxR/BW25113), mutated Receiver (1point mutation/BW25113) in liquid media for 12 h at 37℃.
- Adjust the cell density and add AHL (final concentration, 0, 0.1, 1, 5, 10, 100, and 1000 nM)
- Incubate for 1h at room temperature
- Spindown cells and GFP folurescent level checked
Result
- Both wild type and 1point mutation luxR express GFP under 5nM AHL concentration.
- 2point mutation(I45F,S116A) luxR Receiver express GFP without AHL.
Discussion
This table is quoted from paper.
In the paper, DH5α strain was used.
But wild type luxR in our study expressed GFP under 5nM AHL concentration.
Assumption:There is some difference between strains. Required to do same test on other strains
3.Localizing AHL
Design1
![]()
BBa I729006
In addition to the receiver unit, there added AiiA under the control of lac promoter.
AiiA degrades/ inactivates AHL, lowering the AHL concentration in the cell.
This way cells installed with this circuit can respond, but less sensitive, to the sender cells.
Experiment
- Inoculate E.coli carries this aiiA receiver plasmid in Liquid Media.
- At the same time, inoculate AHL sender E.coli in Liquid Media.
- Dilute receiver cells and spread on agar plate, and spot sender 1μL. Incubate at 37℃ 12hour
- Check plate.(GFP expressed?)
Result
GFP expression was not observed on the plate of aiiA receiver.
Discussion
We thought expression of aiiA significantly decrease the cellular level of AHL. It turned out to be true.
However, it went too much. It does not respond to senders anymore, probably due to too fast AHL degradation.
We need more controlled/ moderate expression of aiiA.
Design2
![]()
This gene circuit is expected so that aiiA is synthesized only when the receiver senses AHL signal. If so, the whole Bacteria Marimo becomes AHL quencher. In fact, the bacteria inside of the Marimo degrades AHL and the AHL diffusion outside of Marimo is inhibited.
Experiment
- Inoculate E.coli carries this aiiA receiver plasmid in Liquid Media.
- At the same time, inoculate AHL sender E.coli in Liquid Media.
- Dilute receiver cells and spread on agar plate, and spot sender 1μL. Incubate at 37℃ 12hour
- Put plate 30℃
- Check GFP expression every 1hour.
Result
- When we compared the GFP expression with the test tube of BBa_T9002, the bacteria with the consructed gene circuit did not fluoresce immediately after incubation, however fluoresced in 18~20 hour.
Discussion
- GFP expression means plux is activated. So we could make moderate aiiA receiver.
- But we need more twisted circuit.
Design3
![]()
Above picture describes aiiA is regulated inverted lux promoter with CI inverter.
- High AHL concentration: no aiiA expression, no AHL degrade.
- Low AHL concentration: aiiA expressed, AHL degraded.
Experiment
We could not finish assembling this part. It will be our future work.
Subpart:BBa_S03840