Edinburgh/DivisionPopper/References
From 2007.igem.org
JoeRokicki (Talk | contribs) |
JoeRokicki (Talk | contribs) |
||
| (32 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
'''MENU''' :[[Edinburgh/DivisionPopper| Introduction]] | [[Edinburgh/DivisionPopper/References|Background]] | [[Edinburgh/DivisionPopper/Applications|Applications]] | [[Edinburgh/DivisionPopper/Design|Design&Implementation]] | [[Edinburgh/DivisionPopper/Modelling|Modelling]] | [[Edinburgh/DivisionPopper/Status|Wet Lab]] | [[Edinburgh/DivisionPopper/SBApproach|Synthetic Biology Approach]] | [[Edinburgh/DivisionPopper/Conclusions|Conclusions]] | '''MENU''' :[[Edinburgh/DivisionPopper| Introduction]] | [[Edinburgh/DivisionPopper/References|Background]] | [[Edinburgh/DivisionPopper/Applications|Applications]] | [[Edinburgh/DivisionPopper/Design|Design&Implementation]] | [[Edinburgh/DivisionPopper/Modelling|Modelling]] | [[Edinburgh/DivisionPopper/Status|Wet Lab]] | [[Edinburgh/DivisionPopper/SBApproach|Synthetic Biology Approach]] | [[Edinburgh/DivisionPopper/Conclusions|Conclusions]] | ||
| - | Some of the biological mechanisms at the base of the Division PoPper design have been widely studied in the past and well characterized in literature (for example, the repression mechanism of Tet and lambda promoters). Others, however, are quite new and only partially understood. In particular, this is true for the ''dif'' recombinase | + | Some of the biological mechanisms at the base of the Division PoPper design have been widely studied in the past and well characterized in literature (for example, the repression mechanism of Tet and lambda promoters). Others, however, are quite new and only partially understood. In particular, this is true for the ''dif'' recombinase Edinbusystem and its function resolving chromosome dimers. Given the incomplete understanding of this system as well as the novel context into which we are placing it, we were forced to make many assumptions during the design process. How will the dif recombination system react to inverting rather than excising DNA? How will shifting the system from the chromosome onto the plasmid effect the recombination system? How many inversion events should we expect per cell division? While the current literature sheds light on many of these questions, there is no substitute for an experimental answer. If the ''dif'' recombination system is to be utilized in synthetic biological constructs it must first be characterized as a device. Our project, and in particular the realization and analysis of the proof of concept system, characterizes this recombination system and its relation to cell division from a synthetic biology perspective and creates a basis for further investigations as well as a device for future constructs. In this section, we propose a brief literature review of the dif recombinase system and its functions in cell division in order to motivate and understand the results of the proof of concept device. |
__TOC__ | __TOC__ | ||
| - | == | + | ==An Analytical Review of the Dif Recombinase System== |
| - | == | + | ===Introduction=== |
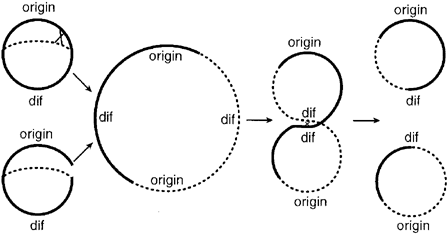
| - | + | In addition to the requirement of replicating all genomic DNA once, and only once, per cell cycle and insuring that exactly one copy of each genome is distributed to the two daughter cells, E. Coli cells must also contend with the problem of chromosome dimers. Dimers form when newly replicated DNA undergoes homologous recombination with its sister chromotid. When this event occurs, the two sister chromotids link together to form one large dimer ring. In normal growth, this event occurs with a probability of about 17% per generation. [Steiner 1998 Jan] | |
| - | + | ||
| - | : | + | :::[[Image:Edinbugh_natural_Xer_recombinase.gif]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | : | + | Figure 1: Schematic representation of the mechanisms by which chromosome dimmers arise and are resolved (figure from Steiner 1998 Jan) |
| - | === | + | ===Dif Phenotype=== |
| - | + | ||
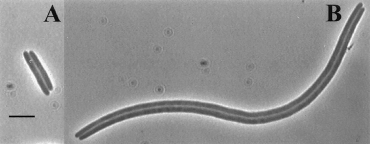
| - | + | A cell without the ability to resolve dimers exhibits what is known as the dif (deletion-induced filamentation) phenotype. Even though the two sister chromosomes are linked, the cell attempts to go about replication as normal. The terminus region (the last part of the genome to undergo replication) is trapped in the vicinity of the septum (a region in the center of the cellular compartment which ultimately pinches off to form the two daughter cells). Without anyway to separate the two genomes, the cell faces one of two outcomes. Either DNA remains trapped in the septum and the cells are tethered together by their genomes and form long filaments, or the septum “guillotines” the DNA resulting in the degradation of both genomes and cell death. [Hendricks 2000] | |
| - | :: | + | |
| - | : | + | [[Image:Difphenotype.gif]] |
| + | |||
| + | Figure 2: Example of cells exhibiting the dif phenotype (from Hendricks 2000) | ||
| + | |||
| + | ===A Model For Resolution=== | ||
| + | |||
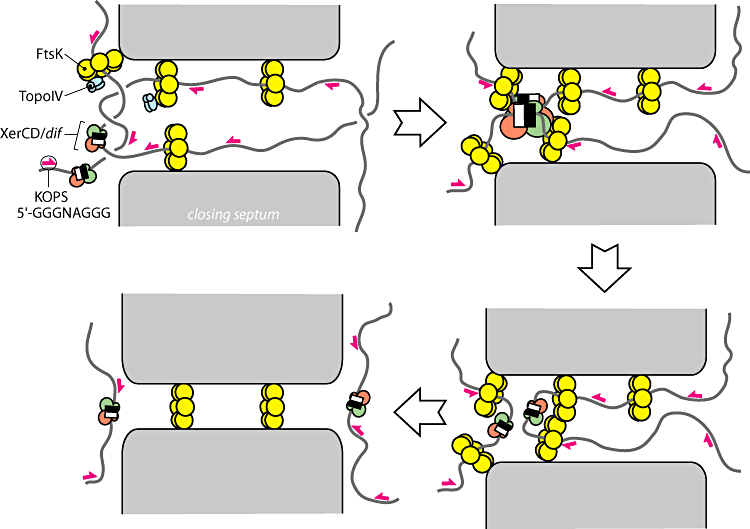
| + | In order to resolve dimers, the cell utilizes two tyrosine recombinases XerC and XerD. These recombinases bind to a 28bp directional sequence known as a dif site. A dimer will contain two dif sites but because they will be at opposite sides of the genome even after they have been bound with recombinase they must somehow be brought into physical proximity for a recombination event to take place. To accomplish this, the cell utilizes the most powerful molecular motor yet discovered [Strick 2006]. Immediately before division, FtsK, assembles into many membrane bound hexamer complexes. These complexes contain a N-terminus region which anchors the complex to the septum and a C-terminus region which functions as a motor. The C-terminus motor preferentially loads onto the DNA at 8bp directional sequences known as KOPS sites [Ptacin 2006]. KOPS sites are littered throughout the DNA near the terminus region like arrows pointing towards the dif sites. The motors utilize this directional information to “reel” the dif sites into the terminus. In this way, the dif sites, which can be many megabases away in terms of sequence, are brought together into close physical proximity. FtsK then works with the dif-bound recombinases to catalyze a recombination event between the two dif sites resolving the chromosome dimer into two identical monomers. | ||
| + | |||
| + | [[Image:Edinbugh_mechanics_of_FtsK.gif]] | ||
| + | |||
| + | Figure 3: Overview of the current model for dimer resolution (From Bigot-2007) | ||
| + | |||
| + | ==A Quantitative Review of the Dif Recombinase System== | ||
| + | |||
| + | In traditional engineering, in order to construct a device, an engineer draws from an inventory of many previously engineered components or modules. The variables that affect the behaviour of these modules as well as detailed descriptions of this behaviour are organized into concise specification sheets attached to each module. | ||
| + | |||
| + | In biological engineering, there is also a vast inventory of heavily characterized modules, but the specifications for these modules are spread out across many hundreds of papers. Biologists sometimes attempt to condense this vast array of information in the form of “reviews.” But often times, the goal of these reviews is to form a readily understandable and unified model of how the device functions in vivo rather than create a resource for engineers who hope to use the device. As a result, in reviews, the information from the materials and methods sections takes a back seat to discussions of possible mechanisms and potential biological implications. | ||
| + | |||
| + | Reviews are designed to facilitate the expansion of our understanding of biology rather than the utilization of what has already been discovered. To facilitate the engineering of biology a quantitative analog to the traditional biological review would be nice. Instead of concepts and models the primary objective would be organizing relevant quantitative information and the conditions associated with it. Below is a rudimentary example of the kind of quantitative information that might be present in this kind of a review. It is populated with information from only two papers, Barre and Bloor. | ||
| + | |||
| + | ===Description=== | ||
| + | The dif recombinase system's primary function is to excise, insert, and invert segments of DNA. Below are a sampling of experimental frequencies for these processes as well as the conditions asocciated with those frequencies. | ||
| + | |||
| + | ===Plasmid=== | ||
| + | Barr 2000 | ||
| + | |||
| + | Substrate with two dif sites (pFC238) | ||
| + | All frequencies after overnight growth | ||
| + | :-30% excision by endogenous ftsK expression | ||
| + | :-70% excision expressing a N-terminal flag tagged ftsK | ||
| + | :-90% excision expressing 818 ftsk | ||
| + | |||
| + | |||
| + | ===Chromosome=== | ||
| + | Barr 2000 | ||
| + | |||
| + | :-Excision of kan from dif-Kan-dif construct within the DAZ is 10 times more likely in RecA+ than RecA- | ||
| + | |||
| + | :-A dif-Kan-dif construct inserted into the chromosome excises with 90%-99% efficiency depending on RecA+/RecA- | ||
| + | |||
| + | Bloor 2006 | ||
| + | |||
| + | :-On the order of 10^-3 or 10^-4 % excision per generation | ||
| + | :-Either 1% or 6.3% excision after 48 hours in E. coli msbB and E. coli ubiB-fadA respectivly | ||
| + | |||
| + | ===Plasmid-Chromosome Interactions=== | ||
| + | Barr 2000 | ||
| + | |||
| + | Substrate with one dif site (pFH127) | ||
| + | |||
| + | :-22.5 times more likely to insert into a dif site in the DAZ than in lacZ | ||
| + | |||
| + | |||
| + | ==References== | ||
| + | |||
| + | Barre, F.-X., M. Aroyo, S. D. Colloms, A. Helfrich, F. Cornet, and D. J. Sherratt. 2000. FtsK functions in the processing of a Holliday junction intermediate during bacterial chromosome segregation. Genes Dev. 14:2976–2988. | ||
| - | + | Bloor AE, Cranenburgh RM. An efficient method of selectable marker gene excision by Xer recombination for gene replacement in bacterial chromosomes. Appl Environ Microbiol. 2006 Apr;72(4):2520-5. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Hendricks EC, Szerlong H, Hill T, Kuempel P. Cell division, guillotining of dimer chromosomes and SOS induction in resolution mutants (dif, xerC and xerD) of Escherichia coli. Mol Microbiol. 2000 May;36(4):973-81. | |
| - | + | Ptacin JL, Nollmann M, Bustamante C, Cozzarelli NR. Identification of the FtsK sequence-recognition domain. Nat Struct Mol Biol. 2006 Nov;13(11):1023-5. Epub 2006 Oct 15. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | Recchia GD, Aroyo M, Wolf D, Blakely G, Sherratt DJ. FtsK-dependent and -independent pathways of Xer site-specific recombination. EMBO J. 1999 Oct 15;18(20):5724-34. | |
| - | + | Steiner WW, Kuempel PL. Cell division is required for resolution of dimer chromosomes at the dif locus of Escherichia coli. Mol Microbiol. 1998 Jan;27(2):257-68. | |
| - | + | ||
| - | + | Steiner WW, Kuempel PL. Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J Bacteriol. 1998 Dec;180(23):6269-75. | |
| - | ::: | + | Strick TR, Quessada-Vial A. FtsK: a groovy helicase. Nat Struct Mol Biol. 2006 Nov;13(11):948-50. Erratum in: Nat Struct Mol Biol. 2007 Jun;14(6):568. |
| - | :: | + | Wang X, Possoz C, Sherratt DJ. Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev. 2005 Oct 1;19(19):2367-77. |
Latest revision as of 03:58, 27 October 2007
MENU : Introduction | Background | Applications | Design&Implementation | Modelling | Wet Lab | Synthetic Biology Approach | Conclusions
Some of the biological mechanisms at the base of the Division PoPper design have been widely studied in the past and well characterized in literature (for example, the repression mechanism of Tet and lambda promoters). Others, however, are quite new and only partially understood. In particular, this is true for the dif recombinase Edinbusystem and its function resolving chromosome dimers. Given the incomplete understanding of this system as well as the novel context into which we are placing it, we were forced to make many assumptions during the design process. How will the dif recombination system react to inverting rather than excising DNA? How will shifting the system from the chromosome onto the plasmid effect the recombination system? How many inversion events should we expect per cell division? While the current literature sheds light on many of these questions, there is no substitute for an experimental answer. If the dif recombination system is to be utilized in synthetic biological constructs it must first be characterized as a device. Our project, and in particular the realization and analysis of the proof of concept system, characterizes this recombination system and its relation to cell division from a synthetic biology perspective and creates a basis for further investigations as well as a device for future constructs. In this section, we propose a brief literature review of the dif recombinase system and its functions in cell division in order to motivate and understand the results of the proof of concept device.
Contents |
An Analytical Review of the Dif Recombinase System
Introduction
In addition to the requirement of replicating all genomic DNA once, and only once, per cell cycle and insuring that exactly one copy of each genome is distributed to the two daughter cells, E. Coli cells must also contend with the problem of chromosome dimers. Dimers form when newly replicated DNA undergoes homologous recombination with its sister chromotid. When this event occurs, the two sister chromotids link together to form one large dimer ring. In normal growth, this event occurs with a probability of about 17% per generation. [Steiner 1998 Jan]
Figure 1: Schematic representation of the mechanisms by which chromosome dimmers arise and are resolved (figure from Steiner 1998 Jan)
Dif Phenotype
A cell without the ability to resolve dimers exhibits what is known as the dif (deletion-induced filamentation) phenotype. Even though the two sister chromosomes are linked, the cell attempts to go about replication as normal. The terminus region (the last part of the genome to undergo replication) is trapped in the vicinity of the septum (a region in the center of the cellular compartment which ultimately pinches off to form the two daughter cells). Without anyway to separate the two genomes, the cell faces one of two outcomes. Either DNA remains trapped in the septum and the cells are tethered together by their genomes and form long filaments, or the septum “guillotines” the DNA resulting in the degradation of both genomes and cell death. [Hendricks 2000]
Figure 2: Example of cells exhibiting the dif phenotype (from Hendricks 2000)
A Model For Resolution
In order to resolve dimers, the cell utilizes two tyrosine recombinases XerC and XerD. These recombinases bind to a 28bp directional sequence known as a dif site. A dimer will contain two dif sites but because they will be at opposite sides of the genome even after they have been bound with recombinase they must somehow be brought into physical proximity for a recombination event to take place. To accomplish this, the cell utilizes the most powerful molecular motor yet discovered [Strick 2006]. Immediately before division, FtsK, assembles into many membrane bound hexamer complexes. These complexes contain a N-terminus region which anchors the complex to the septum and a C-terminus region which functions as a motor. The C-terminus motor preferentially loads onto the DNA at 8bp directional sequences known as KOPS sites [Ptacin 2006]. KOPS sites are littered throughout the DNA near the terminus region like arrows pointing towards the dif sites. The motors utilize this directional information to “reel” the dif sites into the terminus. In this way, the dif sites, which can be many megabases away in terms of sequence, are brought together into close physical proximity. FtsK then works with the dif-bound recombinases to catalyze a recombination event between the two dif sites resolving the chromosome dimer into two identical monomers.
Figure 3: Overview of the current model for dimer resolution (From Bigot-2007)
A Quantitative Review of the Dif Recombinase System
In traditional engineering, in order to construct a device, an engineer draws from an inventory of many previously engineered components or modules. The variables that affect the behaviour of these modules as well as detailed descriptions of this behaviour are organized into concise specification sheets attached to each module.
In biological engineering, there is also a vast inventory of heavily characterized modules, but the specifications for these modules are spread out across many hundreds of papers. Biologists sometimes attempt to condense this vast array of information in the form of “reviews.” But often times, the goal of these reviews is to form a readily understandable and unified model of how the device functions in vivo rather than create a resource for engineers who hope to use the device. As a result, in reviews, the information from the materials and methods sections takes a back seat to discussions of possible mechanisms and potential biological implications.
Reviews are designed to facilitate the expansion of our understanding of biology rather than the utilization of what has already been discovered. To facilitate the engineering of biology a quantitative analog to the traditional biological review would be nice. Instead of concepts and models the primary objective would be organizing relevant quantitative information and the conditions associated with it. Below is a rudimentary example of the kind of quantitative information that might be present in this kind of a review. It is populated with information from only two papers, Barre and Bloor.
Description
The dif recombinase system's primary function is to excise, insert, and invert segments of DNA. Below are a sampling of experimental frequencies for these processes as well as the conditions asocciated with those frequencies.
Plasmid
Barr 2000
Substrate with two dif sites (pFC238) All frequencies after overnight growth
- -30% excision by endogenous ftsK expression
- -70% excision expressing a N-terminal flag tagged ftsK
- -90% excision expressing 818 ftsk
Chromosome
Barr 2000
- -Excision of kan from dif-Kan-dif construct within the DAZ is 10 times more likely in RecA+ than RecA-
- -A dif-Kan-dif construct inserted into the chromosome excises with 90%-99% efficiency depending on RecA+/RecA-
Bloor 2006
- -On the order of 10^-3 or 10^-4 % excision per generation
- -Either 1% or 6.3% excision after 48 hours in E. coli msbB and E. coli ubiB-fadA respectivly
Plasmid-Chromosome Interactions
Barr 2000
Substrate with one dif site (pFH127)
- -22.5 times more likely to insert into a dif site in the DAZ than in lacZ
References
Barre, F.-X., M. Aroyo, S. D. Colloms, A. Helfrich, F. Cornet, and D. J. Sherratt. 2000. FtsK functions in the processing of a Holliday junction intermediate during bacterial chromosome segregation. Genes Dev. 14:2976–2988.
Bloor AE, Cranenburgh RM. An efficient method of selectable marker gene excision by Xer recombination for gene replacement in bacterial chromosomes. Appl Environ Microbiol. 2006 Apr;72(4):2520-5.
Hendricks EC, Szerlong H, Hill T, Kuempel P. Cell division, guillotining of dimer chromosomes and SOS induction in resolution mutants (dif, xerC and xerD) of Escherichia coli. Mol Microbiol. 2000 May;36(4):973-81.
Ptacin JL, Nollmann M, Bustamante C, Cozzarelli NR. Identification of the FtsK sequence-recognition domain. Nat Struct Mol Biol. 2006 Nov;13(11):1023-5. Epub 2006 Oct 15.
Recchia GD, Aroyo M, Wolf D, Blakely G, Sherratt DJ. FtsK-dependent and -independent pathways of Xer site-specific recombination. EMBO J. 1999 Oct 15;18(20):5724-34.
Steiner WW, Kuempel PL. Cell division is required for resolution of dimer chromosomes at the dif locus of Escherichia coli. Mol Microbiol. 1998 Jan;27(2):257-68.
Steiner WW, Kuempel PL. Sister chromatid exchange frequencies in Escherichia coli analyzed by recombination at the dif resolvase site. J Bacteriol. 1998 Dec;180(23):6269-75.
Strick TR, Quessada-Vial A. FtsK: a groovy helicase. Nat Struct Mol Biol. 2006 Nov;13(11):948-50. Erratum in: Nat Struct Mol Biol. 2007 Jun;14(6):568.
Wang X, Possoz C, Sherratt DJ. Dancing around the divisome: asymmetric chromosome segregation in Escherichia coli. Genes Dev. 2005 Oct 1;19(19):2367-77.