Chiba/Making Marimo
From 2007.igem.org
< Chiba(Difference between revisions)
| (49 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
[[Image:chiba_logo.png|center]] | [[Image:chiba_logo.png|center]] | ||
__NOTOC__ | __NOTOC__ | ||
| - | {| style="border:0;width:100%;" cellpadding="20px" cellspacing="0" | + | {| style="border:0;width:100%;font-family:'Trebuchet MS'" cellpadding="20px" cellspacing="0" |
| align="center" | | | align="center" | | ||
| - | [[Chiba|Introduction]] | [[Chiba/Project_Design|Project Design]] ( [[Chiba/Engeneering_Flagella|1. | + | [[Chiba|Home]]<br> |
| + | <span style="font-size:120%;font-weight:bold;">[[Chiba/Introduction|Introduction]] | [[Chiba/Project_Design|Project Design]] ( [[Chiba/Engeneering_Flagella|1.Affinity Tag]] | [[Chiba/Communication|2.Communication Module]] | [[Chiba/Quorum_Sensing|3.Size Control]] ) | [[Chiba/Making Marimo|Making Marimos]] | [[Chiba/Goal|Our Goal]]</span><br> | ||
| + | [[Chiba/Acknowledgements|Acknowledgements]] | [[Chiba/Team_Members|Team Members]] | [http://chem.tf.chiba-u.jp/igem/ iGEM Chiba Website] | [[Chiba/Members_Only|メンバ連絡簿]] | ||
|} | |} | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
==Making Marimos== | ==Making Marimos== | ||
| - | + | [[Image:final.gif|frame|center|'''Fig. 26''' Scheme of final gene cuircuit for Bacteria Marimo.]]<br> | |
==Parts Construction== | ==Parts Construction== | ||
| - | + | Because we found that FliC code includes restriction enzyme(EcoRI,SpeI,PstI) which is used in Biobrick, we divided into plasmid of FliC and one of signal, and aimed the double transformation. | |
| - | Because FliC | + | ===[[Chiba/Flagella/FliC-his_generator|Moving FliC-His generator]]=== |
| - | ===[[Chiba/Flagella/FliC-his_generator|FliC-His generator]]=== | + | ====Experiment==== |
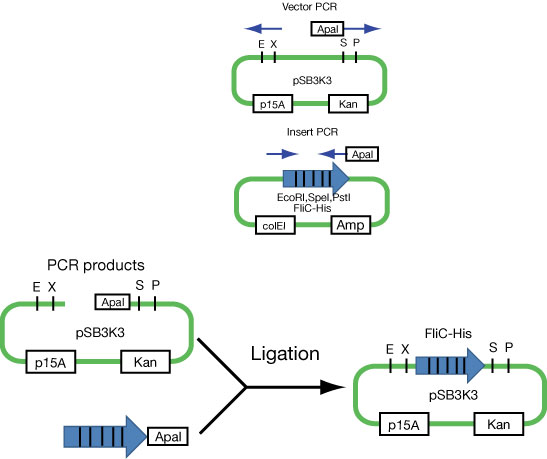
| - | + | [[Image:FliC-His ligation.jpg|frame|'''Fig. 27''' Moving FliC-generators into p15A plasmid (not biobricked yet!)]] | |
| - | We regulate His-tagged FliC by lux promoter. | + | *We regulate the expression of His-tagged FliC by lux promoter. Namely, if LuxR is expressed, bacteria can express FliC ''via'' Quorum Seinsing.<br> |
| - | *Quorum | + | *Because the Quorum Sensing device is on ColE1-type vector, we need to export the FliC unit into the plasmids with compatible origins such as p15A. |
| + | |||
| + | ====Results==== | ||
| + | First attempt to import FliC-His generator into p15A vector (see Fig. 2).<br> | ||
| + | *Communication units are on ColE1 type plasmids. To make this ''stickly FliC'' construct compatible with communication circuits, this is an absolute necessity.....<br> | ||
| + | *Not yet (as of 10/26/2007). The first ligation sucked. Cloning is still underway.<br> | ||
===[[Chiba/Flagella/FliC-His_Biobrick|FliC-his biobrick]]=== | ===[[Chiba/Flagella/FliC-His_Biobrick|FliC-his biobrick]]=== | ||
| - | + | ====Coming Soon==== | |
| - | + | *'''Making Biobrick version of Flic-His and other stickly-FliCs.''' Unfortunately, Many forbidden sites for Biobrick production throughout the FliC gene. We are now eliminating them one by one by site-directed mutagenesis (ExSite PCR method). | |
| - | * | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 05:23, 27 October 2007
|
Home |
Making Marimos
Parts Construction
Because we found that FliC code includes restriction enzyme(EcoRI,SpeI,PstI) which is used in Biobrick, we divided into plasmid of FliC and one of signal, and aimed the double transformation.
Moving FliC-His generator
Experiment
- We regulate the expression of His-tagged FliC by lux promoter. Namely, if LuxR is expressed, bacteria can express FliC via Quorum Seinsing.
- Because the Quorum Sensing device is on ColE1-type vector, we need to export the FliC unit into the plasmids with compatible origins such as p15A.
Results
First attempt to import FliC-His generator into p15A vector (see Fig. 2).
- Communication units are on ColE1 type plasmids. To make this stickly FliC construct compatible with communication circuits, this is an absolute necessity.....
- Not yet (as of 10/26/2007). The first ligation sucked. Cloning is still underway.
FliC-his biobrick
Coming Soon
- Making Biobrick version of Flic-His and other stickly-FliCs. Unfortunately, Many forbidden sites for Biobrick production throughout the FliC gene. We are now eliminating them one by one by site-directed mutagenesis (ExSite PCR method).