Duke/Projects/bc
From 2007.igem.org
EricJosephs (Talk | contribs) |
EricJosephs (Talk | contribs) |
||
| (One intermediate revision not shown) | |||
| Line 11: | Line 11: | ||
==Results== | ==Results== | ||
| - | Both standard assembly and PCR failed to isolate the construct, indicating that there may have been mutations introduced in the restriction sites. Attempts at co-transformation produced little sensitivity to incandescent lights, due to plasmid incompatibility. A majority of the summer was spent examining the possibility of using a red luciferase alternative. The intensity was too low for our purposes. Below is a figure of induced RFP at the beginning of the experiment and seven hours later | + | Both standard assembly and PCR failed to isolate the construct, indicating that there may have been mutations introduced in the restriction sites. Attempts at co-transformation produced little sensitivity to incandescent lights, due to plasmid incompatibility. A majority of the summer was spent examining the possibility of using a red luciferase alternative. The intensity was too low for our purposes. Below is a figure of induced RFP at the beginning of the experiment and seven hours later: |
| - | [[Image: | + | [[Image:t0h.jpg]] [[Image:t7h.jpg]] |
If the signal of the constructs are intense enough and can propagate in a population of E. coli, future work will consist of testing for the light transfer and growth in optical fibers. This can extend the range of signaling, and compartmentalized cells can allow for the operation of more complex logic gates. | If the signal of the constructs are intense enough and can propagate in a population of E. coli, future work will consist of testing for the light transfer and growth in optical fibers. This can extend the range of signaling, and compartmentalized cells can allow for the operation of more complex logic gates. | ||
[[Image:picture1.jpg]] | [[Image:picture1.jpg]] | ||
Latest revision as of 02:08, 27 October 2007
Contents |
Bacterial communication with light
Background and Motivation
Previous attempts towards synchronizing genetic circuits with quorum sensing show that a transition from an unsynchronized to a synchronized regime exists as the strength of coupling increases. Light, as a signal, is more sensitive and versatile than small molecules, and quickly degrades and diffuses. We attempted to characterize a photonic alternative to quorum sensing. With the foundations of an engineered light sensing E. coli for bacterial photography ([http://parts2.mit.edu/wiki/index.php/UT_Austin_2005 UT Austin iGEM 2005], [http://parts2.mit.edu/wiki/index.php/University_of_Texas_2006 2006]), we are characterizing the ability for excited red fluorescent protein to activate photoresponsive systems in nearby bacteria.
Parts and setup
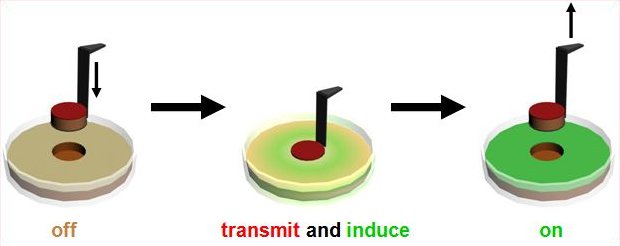
The BBa_M30010 and BBa_M30109 constructs allow for the characterization of signal propagation in a population of E. coli expressing both the light sender and light receiver devices tested in an apparatus as shown below:
The first construct will consist of OmpC (BBa_R0082) regulated expression of λcI with a following PcI regulated mCherry expression. The addition of a repressor gene negates the logic of the red light repressed OmpC promoter, allowing for the light induced expression of mCherry. There was difficulty in isolating the dual regulation light responsive system (BBa_M30109), containing Pbad regulated phycobilins (ho1 and pcyA) followed by a Ptet regulated light sensor (cph8).
Results
Both standard assembly and PCR failed to isolate the construct, indicating that there may have been mutations introduced in the restriction sites. Attempts at co-transformation produced little sensitivity to incandescent lights, due to plasmid incompatibility. A majority of the summer was spent examining the possibility of using a red luciferase alternative. The intensity was too low for our purposes. Below is a figure of induced RFP at the beginning of the experiment and seven hours later:
If the signal of the constructs are intense enough and can propagate in a population of E. coli, future work will consist of testing for the light transfer and growth in optical fibers. This can extend the range of signaling, and compartmentalized cells can allow for the operation of more complex logic gates.