Nhu Nguyen Notebook
From 2007.igem.org
| (43 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
[[Template:BerkiGEM2007_nhuSequencingFiles | My Sequencing Files]]<br> | [[Template:BerkiGEM2007_nhuSequencingFiles | My Sequencing Files]]<br> | ||
<br> | <br> | ||
| - | '''[[User:Nnguyen|Nnguyen]] 15:40, 25 June 2007 (EDT)''' | + | <font/><font color=green><center/><b/>Master Plan</b></center></font> |
| + | Lefty Righty Lefty Righty Lefty Righty | ||
| + | Promoter pTet rbs-ThpA rbs-ThpB rbs-ThpC rbs-ThpD TT | ||
| + | |||
| + | Lefty Lefty Righty | ||
| + | pTet-rbs-ThpA rbs-ThpB-rbs-ThpC rbs-ThpD-TT | ||
| + | |||
| + | Lefty Righty | ||
| + | pTet-rbs-ThpA rbs-ThpB-rbs-ThpC-rbs-ThpD-TT | ||
| + | |||
| + | entire operon | ||
| + | pTet-rbs-ThpA-rbs-ThpB-rbs-ThpC-rbs-ThpD-TT | ||
| + | |||
| + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 15:40, 25 June 2007 (EDT)'''</font> | ||
---- | ---- | ||
Today I came in and I started my tutorial on internal sites because I missed a week of work last week. I completed the tutorial, and I was given my task for the week. Chris gave me a template to work on. The gene is called Streptomyces chrysomallus. There are four genes of interest in the template, and I had to make oligos for these genes. I had to construct two oligos for each gene, a forward primer and a reverse primer. In order to construct the oligos, I used the enzymes BglII and XhoI. BglII was used for the forward primer and XhoI was used for the reverse primer. In 2 out 4 genes, there were internal sites which meant that I had to make additional oligos.For gene ThpA, EcoRI was within it and so I had to make a primer that would remove it. The sequence for EcoRI was gaattc, and I changed it to gagttc. gag and gaa both code for the same amino acid which is glutamic acid. XhoI was within gene ThpB, and again I had to make a primver that would remove the restriction enzyme. <br> | Today I came in and I started my tutorial on internal sites because I missed a week of work last week. I completed the tutorial, and I was given my task for the week. Chris gave me a template to work on. The gene is called Streptomyces chrysomallus. There are four genes of interest in the template, and I had to make oligos for these genes. I had to construct two oligos for each gene, a forward primer and a reverse primer. In order to construct the oligos, I used the enzymes BglII and XhoI. BglII was used for the forward primer and XhoI was used for the reverse primer. In 2 out 4 genes, there were internal sites which meant that I had to make additional oligos.For gene ThpA, EcoRI was within it and so I had to make a primer that would remove it. The sequence for EcoRI was gaattc, and I changed it to gagttc. gag and gaa both code for the same amino acid which is glutamic acid. XhoI was within gene ThpB, and again I had to make a primver that would remove the restriction enzyme. <br> | ||
| Line 12: | Line 25: | ||
| - | '''[[User:Nnguyen|Nnguyen]] 15:40, 26 June 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 15:40, 26 June 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 19: | Line 32: | ||
| - | '''[[User:Nnguyen|Nnguyen]] 23:57, 26 June 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 23:57, 26 June 2007 (EDT)'''</font> |
---- | ---- | ||
At around 2:00, the oligos that I ordered yesterday arrived. I added water to each tube because the primers came in a form of powder. On each tube, it tells how much powder it is in nanomol. I multiplied the number of nanomol by 10 and that was how much water I added. For example, I added 385 microL of water into nn001.<br> | At around 2:00, the oligos that I ordered yesterday arrived. I added water to each tube because the primers came in a form of powder. On each tube, it tells how much powder it is in nanomol. I multiplied the number of nanomol by 10 and that was how much water I added. For example, I added 385 microL of water into nn001.<br> | ||
<br> | <br> | ||
| - | nn001: 38.5 nmol | + | nn001: 38.5 nmol |
| - | nn002: 27.5 nmol | + | nn002: 27.5 nmol |
| - | nn003: 29.0 nmol | + | nn003: 29.0 nmol |
| - | nn004: 29.4 nmol | + | nn004: 29.4 nmol |
| - | nn005: 37.7 nmol | + | nn005: 37.7 nmol |
| - | nn006: 33.6 nmol | + | nn006: 33.6 nmol |
| - | nn007: 26.5 nmol | + | nn007: 26.5 nmol |
| - | nn008: 29.9 nmol | + | nn008: 29.9 nmol |
| - | nn009: 27.5 nmol | + | nn009: 27.5 nmol |
| - | nn010: 31.3 nmol | + | nn010: 31.3 nmol |
<br> | <br> | ||
The DNA sequences for each of primers are: <br> | The DNA sequences for each of primers are: <br> | ||
| - | nn001:cgtacAGATCTatgaccgccgcaccagcagattttgcccgtgcccgaagcgaGttcctcagtatcg | + | nn001:cgtacAGATCTatgaccgccgcaccagcagattttgcccgtgcccgaagcgaGttcctcagtatcg |
| - | nn002:cgcatctcgagtcaggtggcgaacgggccta | + | nn002:cgcatctcgagtcaggtggcgaacgggccta |
| - | nn003:ctagaAGATCTgtgaccatcaccccgcccgc | + | nn003:ctagaAGATCTgtgaccatcaccccgcccgc |
| - | nn004:cgtcactcgagtcaggccgtttcgcggacgc | + | nn004:cgtcactcgagtcaggccgtttcgcggacgc |
| - | nn005:ggttcgagcggctcctTgaggaccagggctccggac | + | nn005:ggttcgagcggctcctTgaggaccagggctccggac |
| - | nn006:gtccggagccctggtcctcAaggagccgctcgaacc | + | nn006:gtccggagccctggtcctcAaggagccgctcgaacc |
| - | nn007:ctagtAGATCTgtgatcgtccgatcgttcag | + | nn007:ctagtAGATCTgtgatcgtccgatcgttcag |
| - | nn008:gtctactcgagtcaggcctcctcggtcagca | + | nn008:gtctactcgagtcaggcctcctcggtcagca |
| - | nn009:cggatAGATCTatgaccaccgaagtacgcgc | + | nn009:cggatAGATCTatgaccaccgaagtacgcgc |
| - | nn010:gtctactcgagtcaggcctcctcggtcagca | + | nn010:gtctactcgagtcaggcctcctcggtcagca |
<br> | <br> | ||
After adding water, I vortex them so that all the loose particles would settle down, and the tubes were put on ice. After placing the tubes in ice, I started predicting my PCR product. My predictions are: ThpA=553 bp; ThpB=1285 bp; ThpC=421 bp; ThpD=916 bp.At the end of the day, I put all 10 tubes in the freezer (below 20). | After adding water, I vortex them so that all the loose particles would settle down, and the tubes were put on ice. After placing the tubes in ice, I started predicting my PCR product. My predictions are: ThpA=553 bp; ThpB=1285 bp; ThpC=421 bp; ThpD=916 bp.At the end of the day, I put all 10 tubes in the freezer (below 20). | ||
| - | '''[[User:Nnguyen|Nnguyen]] 14:06, 27 June 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 14:06, 27 June 2007 (EDT)'''</font> |
---- | ---- | ||
Today, I came in and found out that my template has not yet arrived. While I was updating my notebook, I realized that I have the same two primers (nn008 and nn010). nn008 is correct; however, nn010 is incorrect. I just finished putting my correct primer (nn011) on order. | Today, I came in and found out that my template has not yet arrived. While I was updating my notebook, I realized that I have the same two primers (nn008 and nn010). nn008 is correct; however, nn010 is incorrect. I just finished putting my correct primer (nn011) on order. | ||
The correct DNA sequence for nn010 is: <br> | The correct DNA sequence for nn010 is: <br> | ||
| - | nn011:gtcagctcgagctacttcaccggggtgaagt | + | nn011:gtcagctcgagctacttcaccggggtgaagt |
Without nn011, I am still able to continue with my work even if my template arrives today. | Without nn011, I am still able to continue with my work even if my template arrives today. | ||
| Line 68: | Line 81: | ||
| - | '''[[User:Nnguyen|Nnguyen]] 16:14, 28 June 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 16:14, 28 June 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 83: | Line 96: | ||
| - | '''[[User:Nnguyen|Nnguyen]] 19:21, 29 June 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 19:21, 29 June 2007 (EDT)'''</font> |
---- | ---- | ||
I came in today hoping that I can get ThpD to work. I tried three different techniques hoping that PCR would work. The first PCR tube, I used the enzyme phusion and HF buffer 5x and everything else is the same as the previous PCR tubes. The second PCR tube, I used the enzyme phusion and GC buffer 5x. The third PCR tube, I used the enzyme expand and expand buffer 10x. After taking the tubes out of the PCR and gelling it, the tube with expand did not have a band; the tube with phusion and GC buffer had too many bands, and the tube with phusion and HF buffer showed a satisfying band. <br> | I came in today hoping that I can get ThpD to work. I tried three different techniques hoping that PCR would work. The first PCR tube, I used the enzyme phusion and HF buffer 5x and everything else is the same as the previous PCR tubes. The second PCR tube, I used the enzyme phusion and GC buffer 5x. The third PCR tube, I used the enzyme expand and expand buffer 10x. After taking the tubes out of the PCR and gelling it, the tube with expand did not have a band; the tube with phusion and GC buffer had too many bands, and the tube with phusion and HF buffer showed a satisfying band. <br> | ||
| Line 91: | Line 104: | ||
I finished cloning ThpA and ThpC in the agar dishes. Now I am growing them in LB broth. I have 7 LB broth tubes: 5 from ThpA and 2 from ThpC. I pipeted out 35mL of LB Broth and put 5mL in each tube. I labeled each tube ThpA1, ThpA2.....ThpA5, ThpC1, and ThpC2. Before doing anything to these tubes, I need to make a master solution for my colony PCR. <br> | I finished cloning ThpA and ThpC in the agar dishes. Now I am growing them in LB broth. I have 7 LB broth tubes: 5 from ThpA and 2 from ThpC. I pipeted out 35mL of LB Broth and put 5mL in each tube. I labeled each tube ThpA1, ThpA2.....ThpA5, ThpC1, and ThpC2. Before doing anything to these tubes, I need to make a master solution for my colony PCR. <br> | ||
For Colony PCR I need:<br> | For Colony PCR I need:<br> | ||
| - | 21.8 uL water | + | 21.8 uL water |
| - | 2.5 uL buffer | + | 2.5 uL buffer |
| - | .5 dNTPs | + | .5 dNTPs |
| - | .1 uL Taq | + | .1 uL Taq |
| - | .5 uL Forward primer | + | .5 uL Forward primer |
| - | .5 uL reverse primer | + | .5 uL reverse primer |
Total Volume: 25.9 uL<br> | Total Volume: 25.9 uL<br> | ||
Note: the primers are G0001 and Ca998<br> | Note: the primers are G0001 and Ca998<br> | ||
| Line 102: | Line 115: | ||
<br> | <br> | ||
<br> | <br> | ||
| - | '''Goals for Saturday, June 30,2007''' <br> | + | '''<font color=blue>Goals for Saturday, June 30,2007''' </font><br> |
1. gel ThpB (PCR #2)<br> | 1. gel ThpB (PCR #2)<br> | ||
2. PCR clean-up ThpD and ThpB (if PCR works)<br> | 2. PCR clean-up ThpD and ThpB (if PCR works)<br> | ||
| Line 111: | Line 124: | ||
| - | '''[[User:Nnguyen|Nnguyen]] 15:24, 30 June 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 15:24, 30 June 2007 (EDT)'''</font> |
---- | ---- | ||
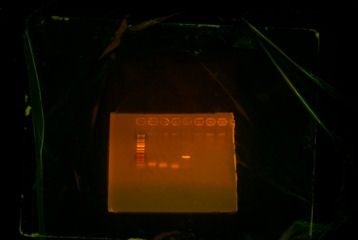
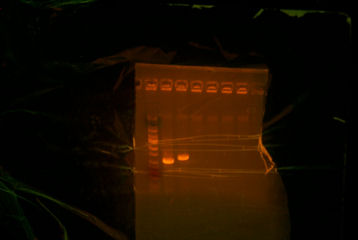
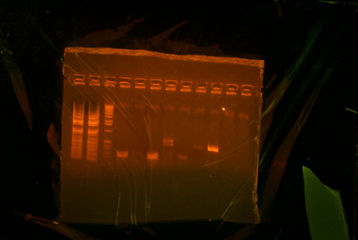
I came in this morning and retrieved my Colony PCR tubes (7) upstairs and my PCR tubes (ThpB PCR #2). I put 5uL of each in a differnt tubes and put .5 uL of buffer in them, and I put them on gel. ThpB turned out satisfying.[[Image:Sewing ThpB.jpg|thumb|The ladder that I used was different from the other ladders. However, the band was satisfying. The band was supposed to be a little above 1000bp and thats what I got.]] <br> | I came in this morning and retrieved my Colony PCR tubes (7) upstairs and my PCR tubes (ThpB PCR #2). I put 5uL of each in a differnt tubes and put .5 uL of buffer in them, and I put them on gel. ThpB turned out satisfying.[[Image:Sewing ThpB.jpg|thumb|The ladder that I used was different from the other ladders. However, the band was satisfying. The band was supposed to be a little above 1000bp and thats what I got.]] <br> | ||
| Line 126: | Line 139: | ||
| - | '''[[User:Nnguyen|Nnguyen]] 00:15, 2 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 00:15, 2 July 2007 (EDT)'''</font> |
---- | ---- | ||
I finished my construction files for all my genes today. <br> | I finished my construction files for all my genes today. <br> | ||
| Line 134: | Line 147: | ||
I prepared a master solution for colony PCR for ThpB and ThpD. I picked 4 colonies for ThpB and ThpD. Altogether, I have 8 PCR tubes, and I also grew them in LB Broth AMP. I put the LB Broth tubes in the shaker for 16 hours. | I prepared a master solution for colony PCR for ThpB and ThpD. I picked 4 colonies for ThpB and ThpD. Altogether, I have 8 PCR tubes, and I also grew them in LB Broth AMP. I put the LB Broth tubes in the shaker for 16 hours. | ||
| - | '''[[User:Nnguyen|Nnguyen]] 17:19, 3 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 17:19, 3 July 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 141: | Line 154: | ||
As for ThpA, I decided to pick 5 (ThpA1a, ThpA2a....ThpA5a)new colonies to grow in broth, and I colony PCR them today. | As for ThpA, I decided to pick 5 (ThpA1a, ThpA2a....ThpA5a)new colonies to grow in broth, and I colony PCR them today. | ||
| - | '''[[User:Nnguyen|Nnguyen]] 00:08, 5 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 00:08, 5 July 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 152: | Line 165: | ||
I chose 10 more colonies from my plate from ThpB/9145. I was unable to grow them in culture tubes because the tubes weren't available during the time. This time, I twirled the colony in the PCR tube and then marked an "X" on a different plate. | I chose 10 more colonies from my plate from ThpB/9145. I was unable to grow them in culture tubes because the tubes weren't available during the time. This time, I twirled the colony in the PCR tube and then marked an "X" on a different plate. | ||
| - | '''[[User:Nnguyen|Nnguyen]] 18:28, 6 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 18:28, 6 July 2007 (EDT)'''</font> |
---- | ---- | ||
I came in today and I retrieved my PCR colony tubes (10 from ThpB) and my PCR tubes (ThpBc, ThpBIc). After gelling them, out of the 10 PCR colony tubes 2 had satisfying bands. ThpBc and ThpBIc had satisfying bands. After seeing that the bands were good, I gelled the entire volume of ThpBc and ThpBIc. After gelling them , I gel extracted them and cleaned them up. After cleaning, ThpBc was ready for its second PCR (sewing). Instead of using expand, I used phusion with the addition of Dmso 100%. There are two tubes for PCR #2 because while I was gel extracting, there were two bands for ThpBc, and I extracted both of them because they were within the range that I wanted. After PCR was done, I gelled and there weren't any bands. I tried sewing once more;however, this time I used expand, and it still didn't work.<br> | I came in today and I retrieved my PCR colony tubes (10 from ThpB) and my PCR tubes (ThpBc, ThpBIc). After gelling them, out of the 10 PCR colony tubes 2 had satisfying bands. ThpBc and ThpBIc had satisfying bands. After seeing that the bands were good, I gelled the entire volume of ThpBc and ThpBIc. After gelling them , I gel extracted them and cleaned them up. After cleaning, ThpBc was ready for its second PCR (sewing). Instead of using expand, I used phusion with the addition of Dmso 100%. There are two tubes for PCR #2 because while I was gel extracting, there were two bands for ThpBc, and I extracted both of them because they were within the range that I wanted. After PCR was done, I gelled and there weren't any bands. I tried sewing once more;however, this time I used expand, and it still didn't work.<br> | ||
| Line 158: | Line 171: | ||
For ThpCb and ThpA, I picked 8 colonies from each plate and grew them in culture tubes and colony PCR them. After Colony PCR, I ran them on gel and there were bands and ThpCb seemed to have the desired band. After choosing 3 samples for ThpCb (ThpCb3,ThpCb6,ThpCb8) and ThpA(ThpA3b, ThpA6b, ThpA8b), I miniprepped along with 2 samples from ThpB. | For ThpCb and ThpA, I picked 8 colonies from each plate and grew them in culture tubes and colony PCR them. After Colony PCR, I ran them on gel and there were bands and ThpCb seemed to have the desired band. After choosing 3 samples for ThpCb (ThpCb3,ThpCb6,ThpCb8) and ThpA(ThpA3b, ThpA6b, ThpA8b), I miniprepped along with 2 samples from ThpB. | ||
| - | '''[[User:Nnguyen|Nnguyen]] 01:40, 9 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 01:40, 9 July 2007 (EDT)'''</font> |
---- | ---- | ||
I sent ThpCb3, ThpCb6, ThpCb8; ThpA3b, ThpA6b, ThpA8b, ThpB4a, ThpB5a to sequencing. For ThpB, I needed to add the ca998 and G01001 because it has 1200 base pairs. <br> | I sent ThpCb3, ThpCb6, ThpCb8; ThpA3b, ThpA6b, ThpA8b, ThpB4a, ThpB5a to sequencing. For ThpB, I needed to add the ca998 and G01001 because it has 1200 base pairs. <br> | ||
| Line 164: | Line 177: | ||
For ThpBc, I tried sewing the two parts together. However, this time I only have one tube because I did not have enough of the part with the internal site.<br> | For ThpBc, I tried sewing the two parts together. However, this time I only have one tube because I did not have enough of the part with the internal site.<br> | ||
| - | '''[[User:Nnguyen|Nnguyen]] 15:35, 10 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 15:35, 10 July 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 173: | Line 186: | ||
Clone Savor: I took 10uL the broth media that I had of ThpCb6 and ThpD2 pipetted unto a piece of paper (clone saver. | Clone Savor: I took 10uL the broth media that I had of ThpCb6 and ThpD2 pipetted unto a piece of paper (clone saver. | ||
| - | '''[[User:Nnguyen|Nnguyen]] 18:43, 11 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 18:43, 11 July 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 186: | Line 199: | ||
I picked out 8 more colonies from plate ThpA/9145 and colony PCR them. Hopefully after miniprepping them and sending them to sequencing, the sequences will turn on out great. <br> | I picked out 8 more colonies from plate ThpA/9145 and colony PCR them. Hopefully after miniprepping them and sending them to sequencing, the sequences will turn on out great. <br> | ||
| - | '''[[User:Nnguyen|Nnguyen]] 12:38, 12 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 12:38, 12 July 2007 (EDT)'''</font> |
---- | ---- | ||
I came in and took out the liquid media for ThpCb6, ThpD2, and RBS from the incubator. I minipreppred those and got them ready for digestion. For RBS, I had: <br> | I came in and took out the liquid media for ThpCb6, ThpD2, and RBS from the incubator. I minipreppred those and got them ready for digestion. For RBS, I had: <br> | ||
| - | 20uL of water | + | 20uL of water |
| - | 6 uL of plasmid | + | 6 uL of plasmid |
| - | 3uL 10x buffer NEBuffer #2 | + | 3uL 10x buffer NEBuffer #2 |
| - | .5uL of BamHI | + | .5uL of BamHI |
| - | .5uL of XhoI. | + | .5uL of XhoI. |
For ThpCb6 and ThpD, everything is the same as RBS except for the enzymees that I used. I used BglII and XhoI for ThpCb6 and ThpD2. The total volume for my digestion tubes was 30uL. The tubes were put in the incubator for 2 hours. After digestion, I prepared the tubes for ligation. The duration for ligation was 30 minutes to an hour. In the ligtaion tubes, I had:<Br> | For ThpCb6 and ThpD, everything is the same as RBS except for the enzymees that I used. I used BglII and XhoI for ThpCb6 and ThpD2. The total volume for my digestion tubes was 30uL. The tubes were put in the incubator for 2 hours. After digestion, I prepared the tubes for ligation. The duration for ligation was 30 minutes to an hour. In the ligtaion tubes, I had:<Br> | ||
| - | 4uL water | + | 4uL water |
| - | 3uL insert (smaller piece-->ThpCb6,ThpD2) | + | 3uL insert (smaller piece-->ThpCb6,ThpD2) |
| - | 1uL vesctor (larger piece-->RBS) | + | 1uL vesctor (larger piece-->RBS) |
| - | 1uL ligase buffer | + | 1uL ligase buffer |
| - | 1uL ligase | + | 1uL ligase |
The total volume for ligation wa 10uL.<br> | The total volume for ligation wa 10uL.<br> | ||
After ligation was finished, I had to heat kill the ligase for 15-20 minutes. After heat kill, I digested the ligation product with BamHI and BglII. The volume for the digested ligation tubes was 20uL. In the tubes I had: <br> | After ligation was finished, I had to heat kill the ligase for 15-20 minutes. After heat kill, I digested the ligation product with BamHI and BglII. The volume for the digested ligation tubes was 20uL. In the tubes I had: <br> | ||
| - | 10uL ligation product | + | 10uL ligation product |
| - | 7uL water | + | 7uL water |
| - | 2uL buffer #2 | + | 2uL buffer #2 |
| - | .5uL BamHI | + | .5uL BamHI |
| - | .5uL BglII | + | .5uL BglII |
After digestion, I got ready for transfomation. Instead of using competent cells, I used the lefty and righty cells. I had to grow RBS-ThpCb6 into a lefty strain, and I grew RBS-ThpD2 into a righty strain. I put the dishes into the incubator for 16 hours.<br> | After digestion, I got ready for transfomation. Instead of using competent cells, I used the lefty and righty cells. I had to grow RBS-ThpCb6 into a lefty strain, and I grew RBS-ThpD2 into a righty strain. I put the dishes into the incubator for 16 hours.<br> | ||
<br> | <br> | ||
ThpA has been having some problems with sequencing because the sequences haven't been matching up very well. In order to fix this problem, I carried out the process of ligation again for ThpA. <br> | ThpA has been having some problems with sequencing because the sequences haven't been matching up very well. In order to fix this problem, I carried out the process of ligation again for ThpA. <br> | ||
| - | '''[[User:Nnguyen|Nnguyen]] 13:00, 13 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 13:00, 13 July 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 222: | Line 235: | ||
Since there were good colonies for ThpA, I picked 10 colonies and PCR colony them, and I also grew them in culture tubes for 16 hours in the incubator. | Since there were good colonies for ThpA, I picked 10 colonies and PCR colony them, and I also grew them in culture tubes for 16 hours in the incubator. | ||
| - | '''[[User:Nnguyen|Nnguyen]] 13:06, 14 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 13:06, 14 July 2007 (EDT)'''</font> |
---- | ---- | ||
| Line 229: | Line 242: | ||
I gelled the Colony PCR products for ThpA and found out that 4 (3,4,5,9 worked) out of 10 colonies had the desired bands. I miniprepped those along with 10 samples for ThpCb6 (5 samples) and ThpD2 (5 samples). | I gelled the Colony PCR products for ThpA and found out that 4 (3,4,5,9 worked) out of 10 colonies had the desired bands. I miniprepped those along with 10 samples for ThpCb6 (5 samples) and ThpD2 (5 samples). | ||
| - | '''[[User:Nnguyen|Nnguyen]] 15:29, 16 July 2007 (EDT)''' | + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 15:29, 16 July 2007 (EDT)'''</font> |
| + | ---- | ||
| + | |||
| + | I retrieved my culture tubes and miniprepped them (5 tubes of ThpB5a). <br> | ||
| + | I sent RBS-ThpCb6-4(lefty), RBS-ThpD2-5 (righty), RBS-ThpB5a3 (righty) to sequencing today. i also sent the 4 samples of ThpA to sequencing today.<br> | ||
| + | I prepared to ligate my composite parts for ThpC and ThpD today; however, I am going to stop after the first digestion because I have to leave early today.<br> | ||
| + | For RBS-ThpCb6, I will use BamHI an XhoI. By using BamHI and XhoI, BglII will get methylated. After digestion is completed, I will gel it and cut out the larger band. For RBS-ThpD2, I will use BglII and XhoI. By using BglII and XhoI, BamHI will get methylated. After digestion is completed, I will gl it and cut out the smaller band. After cutting it, I will clean up my digestion product. | ||
| + | |||
| + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 12:50, 17 July 2007 (EDT)'''</font> | ||
| + | ---- | ||
| + | |||
| + | Since RBS-ThpCb64 and RBS-ThpD25 didn't work after digestion. I'm planned to try another digestion and send that to sequencing to see whether if it matches up with what I want. I digested RBS-ThpCb61 and RBS-ThpD22. The predicted band for RBS-ThpCb61 is 2468bp, and the predicted band for RBS-ThpD22 is 900 bp. After gelling it, the band for RBS-ThpCb61 seems as if it was closer to 2000bp rather than 2400bp. There was only one band for RBS-ThpD22. There were supposed to be two bands because I was supposed to extract the smaller band. <br> | ||
| + | Seeing that the digestions aren't working, I choose 10 more colonies from each dish and colony PCR them as well as growing them in broth. I gelled the colony PCR products and ThpCb6 had a band but it was a lot smaller than what was expected. ThpD2 did not have any band at all. <br> | ||
| + | <br> | ||
| + | The sequences arrived from Quintara and in all the samples for ThpA, there was at least one point mutation. In conclusion, John and I decided that we should try a quickchange and change the guanine to an adenine (this happened in sample ThpA3). The amino acid that I have is glycine(GGG) and the desired amino acid is glutamic acid (GAG). I order two new primers yesterday. The forward primer is 51 base pairs (25bp before the adenine and 25bp after adenine). The reverse primer is the reverse complimentary of the forward primer. | ||
| + | |||
| + | |||
| + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 13:02, 18 July 2007 (EDT)'''</font> | ||
| + | ---- | ||
| + | |||
| + | I came in today and miniprepped 2 samples of ThpCb6 (RBS-ThpCb67a, RBS-ThpCb69a) and ThpD2(RBS-ThpD23a, RBS-ThpD26a. These are the samples I colony PCR yesterday. I also miniprepped 8 samples of ots AB (lefty) for Vinni.<br> | ||
| + | <br> | ||
| + | I checked my sequences for RBS-ThpCb64 and RBS-ThpD25 and they matched up perfectly. I am going to try to redigest using the same plasmids since the sequences matched up perfectly. After digestion was finished, I gelled it and I extracted the desired bands. For ThpCb, I extracted the larger band (2400) and for ThpD2, I extracted the smaller band (900). The larger band for ThpD2 was about 2000. After gel extraction, I cleaned it. After cleaning it, I ligated the two composite parts together. I heat kill for 15 minutes afterwards and then transformation. I transformed the composite parts into righty.<br> | ||
| + | <br> | ||
| + | At the end of the day, I realized I didn't send all of my RBS-ThpB5a samples to sequencing and so I prepared tubes for sequencing. I sent 4 different samples (ThpB5a1,2,4,5). | ||
| + | |||
| + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 14:46, 20 July 2007 (EDT)</font> | ||
| + | ---- | ||
| + | |||
| + | The sequences came back for RBS-ThpB5, and it seems as if all 4 samples have the the same mutation (an additional mutation). We decided to send one of the sequences for RBS-ThpB5a to campus so they can sequence it.<br> | ||
| + | 3.2 uL primer | ||
| + | 5uL plasmid | ||
| + | 4.8uL water | ||
| + | Total Volume: 13uL | ||
| + | <br> | ||
| + | * The oligos I ordered for ThpA3 came. <br> | ||
| + | NN012 (forward): tcgatgcgccacgagtggaggacggagccgcgatctggcgcatagcccgcg | ||
| + | NN013(reverse): cgcgggctatgcgccagatcgcggctccgtcctccactcgtggcgcatcga | ||
| + | |||
| + | <font color=blue><b/>Quickchange</b> </font><br> | ||
| + | 1. set up 2 separate primer extension reactions | ||
| + | 5uL 10x Pfu buffer | ||
| + | 1uL 10um primer | ||
| + | .1-.2 uL plasmid template | ||
| + | 1uL 10uM dNTP | ||
| + | 1uL Pfu turbo polymerase | ||
| + | 41.8uL water | ||
| + | Total Volume: 50uL | ||
| + | |||
| + | 2. Incubate: | ||
| + | 1. 94 degrees: 30secs | ||
| + | 2. 95 degrees: 30secs | ||
| + | 3. 55 degrees: 1 min ( I changed this to 1:30 minutes) | ||
| + | 4. 68 degrees, 2 min/kb (I changed this to 5 minutes) | ||
| + | * I repeated steps 2-4 three times and held it at 4 degrees. Total cycles: 4 | ||
| + | |||
| + | 3. Combine 25uL from each tubes into one tube and add 1uL Pfu polymerase. Incubate (look at previous step) for 18 cycles. | ||
| + | |||
| + | 4. Remove 25uL of the reaction, add 10 units (.5uL) of DpnI enzyme. Mix and incubate at 37 degrees for one hour. | ||
| + | |||
| + | 5. Transform 1uL of reaction into competent cells and plate them. ( I transformed it into lefty cells and then plated them). | ||
| + | <br> | ||
| + | *colony PCR RBS-ThpCb64-RBS-ThpD25, gelled them and miniprepped samples 7,8,9, and 10. | ||
| + | |||
| + | '''<font color=lightblue>[[User:Nnguyen|Nnguyen]] 14:46, 21 July 2007 (EDT)</font> | ||
| + | ---- | ||
| + | |||
| + | I came in today and colony PCR. I had 3 colonies after transforming my quickchange into a lefty. While preparing for colony PCR, I grew them in liquid media as well. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 23 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | |||
| + | I came in and retrieved my culture tubes, and Vinni helped me miniprepped them. I sent those off the Quintara (thpA-->quickchange) | ||
| + | While I was making my Ape files, I realized that my genes didnt have a BamHI site and so we had to figure out a way to insert it in. | ||
| + | * I made 5 oligos (common primer--> have XhoI and 20 bp from plasmid with BamHI site in front of XhoI, and the other 4 oligos will have 20bp from the end of the gene with BamHI right after it--> note: i reverse the oligios) | ||
| + | nn016:gtcagggatcctaaCTCGAGctgcagttgcaggcttcctc (common primer) | ||
| + | nn017:gtcagggatcctcaggtggcgaacgggccta (reverse primer for thpA) | ||
| + | nn018:gtcgaggatcctcaggccgtttcgcggacgc (reverse primer for thpB) | ||
| + | nn019:gtcgaggatcctcaggcctcctcggtcagca (reverse primer for thpC) | ||
| + | nn020:gctgaggatccctacttcaccggggtgaagt (reverse primer for thpD) | ||
| + | |||
| + | * I also order 2 more primers for RBS-ThpB so when I send it off to sequencing, the sequence would show the 3rd mutation. | ||
| + | nn014:ctccggactcaacaagcccg (forward primer) | ||
| + | nn015:gccgtagccgctgatggact (reverse primer) | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 24 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | |||
| + | The sequences for ThpA (quickchange) arrived. Out of 3 samples, one sample worked. <br> | ||
| + | When the primers came in today, I dilute it to 10uM and then prepared for PCR. | ||
| + | 41 uL water | ||
| + | 5 uL buffer 10x | ||
| + | 1 uL Reverse primer (it varies-->depends on the plasmid that I used) | ||
| + | 1 uL dNTPS | ||
| + | .5 plasmid (it varies because I'm using 4 different genes) | ||
| + | .5 expand | ||
| + | Total Volume: 50 uL | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 25 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | |||
| + | After retrieving my PCR tubes, I gelled them and found out that only ThpCb6 and RBS-ThpCb64 worked. The other samples did not have any bands. | ||
| + | *I tried PCR once more but this time I elongated the time to 3 minutes and changed the temperature to 50C. | ||
| + | *Once again, PCR did not work. I tried PCR once more but this time we decided to have two different sets: one with Dmso 100% and one without Dmso 100%. In both sets, I doubled the amount of oligos. Each tubes consists of (there are six different tubes for each set:ThpA3, ThpB5a, ThpC6,ThpD2, rbs-ThpB5a3, rbs-ThpD25): | ||
| + | <u/>Set #1: With Dmso 100%</u> <u/>Set #2: Without Dmso 100%</u> | ||
| + | 34uL water 39uL water | ||
| + | 5uL 10x buffer 5uL 10x buffer | ||
| + | 5uL Dmso 100% 2uL common primer | ||
| + | 2uL common primer 2uL reverse primer(varies) | ||
| + | 2uL reverse primer(varies) 1uL dNTPs | ||
| + | 1uL dNTPs .5uL plasmid (varies) | ||
| + | .5uL plasmid(varies) .5uL expand | ||
| + | .5 expand | ||
| + | Total Volume: 50uL | ||
| + | Since ThpCb6 and rbs-ThpCb64 worked, I cleaned the PCR product and prepared my tubes for digestion. | ||
| + | 20uL water | ||
| + | 6uL PCR product | ||
| + | 3uL buffer #2 | ||
| + | .5uL BamHI | ||
| + | .5uL DpnI | ||
| + | Total Volume: 30uL | ||
| + | *After digestion, I gel extracted them, cleaned them, and then prepared for ligation. | ||
| + | 4uL water | ||
| + | 4uL digested PCR product | ||
| + | 1uL ligase | ||
| + | 1uL ligase buffer | ||
| + | Total Volume: 10uL | ||
| + | *After ligation was transformation. I transformed ThpCb6 into competent cells and rbs-ThpCb64 into lefty. | ||
| + | |||
| + | |||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 26 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | |||
| + | I came in and retrieved my PCR products and gelled them. The samples that consist of Dmso 100% had a bright band except for ThpD2 and rbs-ThpD25. I digested ThpA3, rbs-ThpB, ThpB, rbs-ThpD25, ThpD2. I digested the samples with Dmso 100% except for ThpDs. I gelled the digestion to gel extract; however, there weren't any bands. Due to a problem with the incubator (the temperature was beyond what it was supposed to be), I believe that was the cause of my outcome. I will start all over tomorrow.<br> | ||
| + | <br> | ||
| + | *I colony PCRed 5 colonies from each of the plate that I transformed yesterday (ThpCb6, rbs-ThpCb64-->lefty, rbs-ThpB5a3-->righty (without BamHI)). I also grew them in liquid media. I gelled my colony PCR products and there were bands for all the samples. rbs-ThpB5a3 seemed to have the right band; however, the others didn't. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 27 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | *I miniprepped all of rbs-thpB5a3 (without BamHI) and rbs-ThpCb64-3, rbs-ThpCb64-5, ThpCb6-3, and ThpCb6-5. | ||
| + | *I gel extracted ThpA3 and ThpB5a and rbs-ThpB5a3 {all with BamHI) and then cleaned them up, ligated them, and then transformed them. ThpA3-->Righty, ThpB5a-->Lefty, rbs-ThpB5a3-->Lefty. | ||
| + | *I also transformed rbs-ThpCb645 and rbs-ThpCb643 into righty competent cells. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 28 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | *There were colonies on only rbs-ThpCb645,3. There were one colony for thpA3 and ThpB5a. | ||
| + | *Colony PCRed them and grew them in culture tubes (8-->3 for each rbs-C and one for A and one for B) | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:21, 20 July 2007 (EDT) </font>''' | ||
| + | ---- | ||
| + | *Colony PCR was quite a success. | ||
| + | *miniprepped ThpA3,ThpB5a, rbs-ThpCb645-1,2 and rbs-ThpCb643-1,2. | ||
| + | I PCRed rbs-ThpD2-5 and ThpD2 once more. I made four tubes, one without Dmso 100% and one with Dmso 100%. | ||
| + | <u/>Tube 1 with Dmso 100%:</u> <u/>Tube 2 without Dmso 100%:</u> | ||
| + | 34uL water 39uL water | ||
| + | 5uL 10x buffer 5uL 10x buffer | ||
| + | 5uL Dmso 100% 2uL reverse primers (nn020) | ||
| + | 2uL reverse primer (nn020) 2uL forward primer (ca998) | ||
| + | 2uL forward primer (ca998) 1uL dNTPs | ||
| + | 1uL dNTPs] .5uL template (rbs-ThpD2-5/ThpD2) | ||
| + | .5uL template (rbs-ThpD2-5/ThpD2) .5uL expand | ||
| + | .5uL expand | ||
| + | Total Volume: 50uL | ||
| + | *Test Digestion for ThpA3 and ThpB5a: | ||
| + | 6uL water | ||
| + | 2uL plasmid (ThpA3/ThpB5a) | ||
| + | .5uL BamHI | ||
| + | .5uL BglII | ||
| + | 1uL buffer #2 | ||
| + | Total Volume: 10uL | ||
| + | *Gelled ThpD2, rbs-ThpD2-5, ThpA3 and ThpB5a. There were bands for A and B but there weren't anything for ThpD2 and rbs-ThpD2-5. | ||
| + | *PCR rbs-ThpD2-5 again. This time without Dmso 100% (Farnaz did one tube and I did another).[same contents as previous PCR tubes] | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 31 July 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *Ligated ThpA3 and ThpB5a with BamHI again and then transformed them. | ||
| + | *PCR rbs-ThpD2-5 again. This time we used phusion rather than expand. There are a total of 4 PCR tubes. | ||
| + | <u/>Set 1 (with Dmso 100%):</u> | ||
| + | 29uL water 29uL water | ||
| + | 10uL buffer 5x(HF) 10uL buffer 5x(HF) | ||
| + | 5uL Dmso 100% 5uL Dmso100% | ||
| + | 2uL reverse primer (nn020) 2uL reverse primer (nn020) | ||
| + | 2uL forward primer (ca998) 2uL common primer (nn016) | ||
| + | 1uL dNTPs 1uL dNTPs | ||
| + | .5uL template (rbs-ThpD2-5) .5uL template (rbs-ThpD2-5) | ||
| + | .5uL phusion .5uL phusion | ||
| + | Total Volume: 50uL | ||
| + | |||
| + | <u/>Set 2 (without Dmso100%)</u> | ||
| + | 34uL water | ||
| + | 10uL buffer 5x (HF) | ||
| + | 2uL reverse primer (nn020) | ||
| + | 2uL forward primer/common primer | ||
| + | 1uL dNTPs | ||
| + | .5uL template (rbs-ThpD2-5) | ||
| + | .5uL phusion | ||
| + | Total Volume: 50uL | ||
| + | *Gelled the PCR products and all them have bands except for rbs-ThpD2-5 without Dmso100% with the forward primer. | ||
| + | *Cleaned rbs-ThpD2-5 with Dmso100% with the common primer. | ||
| + | *Overnight digestion: | ||
| + | 24uL water | ||
| + | 20uL cleaned up PCR product (rbs-ThpD2-5) | ||
| + | 1uL BamHI | ||
| + | 5uL BamHI buffer | ||
| + | Total Volume: 50uL | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 1 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *Gelled overnight digestion (rbs-ThpD2-5). Gel extracted the top utmost band, cleaned up, and then ligated. After ligation, I transformed rbs-ThpD2-5 into lefty. | ||
| + | *I colony PCRed 7 colonies from the righty-ThpA3 plate and one colony on the lefty ThpB5a plate. I gelled them and there weren't any bands. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 2 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *Lefty rbs-ThpD2-5 has colonies! I grew 8 colonies in culture tubes. | ||
| + | *Miniprepped 4 our of 7 samples for ThpA3(ThpA3-3,5,6,7) and one for ThpB5a. I sent those samples to sequencing. | ||
| + | *I test digestd my miniprepped samples for two hours and then gelled them. There were bands, however, the bands did not seem right. I digested one more time; however, this time I digested with only BglI, and I used Buffer #3. I also digested rbs-ThpB5a3 along with ThpA3 and ThpB5a. | ||
| + | 11.5uL water | ||
| + | 6uL clean PCR | ||
| + | 2uL 10x Nebuffer 3 | ||
| + | .5uL BglI | ||
| + | Total Volume: 20uL | ||
| + | When digestion was finished, I gelled the samples. Along the samples, I also gelled the clean PCR product to compare the differences. Unfortunately, there were only bands for the clean PCR product and not the digestion. | ||
| + | *The sequences arrived for the PCR product for ThpA3 and rbs-ThpB5a3 and rbs-ThpD2-5. The only sequence that seemed to work was rbs-ThpD2-5. | ||
| + | *Since phusion worked for rbs-ThpD2-5, I tried to use phusion for ThpA3, ThpB5a, and rbs-ThpB5a3 instead of expand. | ||
| + | 29uL water | ||
| + | 10uL buffer 10x (HF) | ||
| + | 5uL Dmso 100% | ||
| + | 2uL specific reverse primer Program used on PCR machine: | ||
| + | 2uL common primer 3K; temperature: 55; elongation time: 3 minutes | ||
| + | 1uL dNTPs | ||
| + | .5uL template (varies) | ||
| + | .5uL phusion | ||
| + | Total volume: 50 uL | ||
| + | *I ordered the correct reverse primers for ThpA3 and ThpB5a. I'm planning to start from the beginning for these two genes unless the PCR tubes with phusion work. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 3 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *Gelled PCR product from yesterday and they all had good bands. I cleaned them and prepared for digestion (2hrs) | ||
| + | *I gelled my digestions and gel extracted them, cleaned them, and prepared for ligation (30mins) | ||
| + | *After ligation, I transformed them. ThpA3-->righty, ThpB5a-->lefty, rbs-ThpB5a3-->lefty | ||
| + | I sent PCR products for ThpA3, ThpB5a, rbs-ThpB5a3 to sequencing (6uL water, 2uL PCR product). | ||
| + | *Ricky miniprepped 8 culture tubes for me for rbs-thpD25 and I sent them all off to sequencing. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 4 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *The sequences came back for rbs-ThpD25, and fortunately, all of the sequences matched up very well. | ||
| + | *The PCR products for ThpA3 matched up very well but the BamHI and XhoI sites didn't match up.As for tbs-ThpB5a3 and ThpB5a, it had the same problem as ThpA3 but everything matched up. | ||
| + | *There were colonies on the plates I transformed yesterday. I grew 5 colonies in culture tubes. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 6 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *miniprepped ThpA3, ThpB5a,rbs-ThpB5a3 (15 total-->5 from each plate) | ||
| + | *after miniprepping, I test digested (2hrs) all my miniprepped samples. | ||
| + | 6uL water | ||
| + | 2uL plasmid | ||
| + | 1uL buffer 2 | ||
| + | .5uL BamHI | ||
| + | .5uL BglII | ||
| + | Total Volume: 10uL | ||
| + | *I digested rbs-ThpD254 with BamHI and EcoRI, and I digested TT with BglII and EcoRI. | ||
| + | <u/>rbs-ThpD254</u> <u/> TT</u> | ||
| + | 20uL water 20uL water | ||
| + | 6uL plasmid 6uL plasmid | ||
| + | 3uL 10x buffer 2 3uL 10x buffer 2 | ||
| + | .5uL BamHI .5uL BglII | ||
| + | .5uL EcoRI .5uL EcoRI | ||
| + | Total Volume: 30uL | ||
| + | |||
| + | *I gel extracted the smaller band for rbs-ThpD254(~923bp), and I gel extracted the larger band for TT. I cleaned up and prepared for ligation (30min-1hr). rbs-ThpD254 is the insert and I put in 3uL. TT is the vector and I put in 1uL. | ||
| + | *After ligation, I heat kill for 15-20minutes and then I digested the ligation product. After digestion, I transformed rbs-ThpD254 into a righty. | ||
| + | *I gelled my test digest and there were bands for all of them but there were desired bands for certain samples so I picked those and send them off to sequencing (ThpA31,ThpA32,ThpA35,ThpB5a1,ThpB5a3,rbs-ThpB5a31,rbs-ThpB5a33 and rbs-ThpB5a35). | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 7 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *The sequences came for ThpA3, ThpB5a, and rbs-ThpB5a3 and they all seemed to matched up well. | ||
| + | *I prepared digestion for ThpA3, T7-rbs, rbs-ThpB5a35, and rbs-ThpCb6432. | ||
| + | *I digested ThpA3 with BamHI and BglII, T7-rbs with XhoI and BamHI, rbs-ThpB5a35 with BamHI and XhoI, and rbs-ThpCb6432 with BglII and XhoI. | ||
| + | * I would need to extract the smaller band for ThpA3 (537bp), the larger band for T7-rbs (2106bp), the larger band for rbs-ThpB5a35 (3340bp), and the smaller band for rbs0ThpCb6432 (428bp). | ||
| + | *Unfortunately when I gelled the digestions, there were bands; however, only some of them were bands that I wanted. I decided to re-digest them. | ||
| + | *After gelling the digestions for the second time, I got them same results. This time, I gel extracted T7-rbs, rbs-ThpB5a35 and rbs-ThpCb6432. ThpA3 had the larger band but it didn't have the smaller band. | ||
| + | *I cleaned up the ones that I gel extracted and prepared for ligation for rbs-B and rbs-C. The vector was rbs-B and the insert was rbs-C. I heat kill afterwards for 15 mins, and then digested the ligation product with BamHI and BglII. I transformed rbs-B-rbs-C into a lefty. | ||
| + | *Before I left, I set an overnight digestion and a test digestion for ThpA3 hoping that it would work. | ||
| + | <u/>Overnight Digestion:</u> <u/>Test Digestion:</u> | ||
| + | 31uL water 6uL water | ||
| + | 12uL plasmid 2uL plasmid | ||
| + | 5uL buffer 2 1uL buffer 2 | ||
| + | 1uL BamHI .5uL EcoRI | ||
| + | 1uL BglII .5uL XhoI | ||
| + | Total Volume: 50uL Total Volume: 10uL | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 8 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *miniprepped 5 culture tubes for rbs-ThpD254-TT. | ||
| + | *Gelled overnight digestion and test digestion. There was only one band for all overnight digestion, and there were two bands for the test digestion. | ||
| + | *Realizing that I had to digest ThpA3 with BglII and XhoI instead of BglII and BamHI, I redid the digestion with the correct enzymes. I then extracted the smaller band which is ~546bp and then I cleaned up the product. | ||
| + | *After clean up, I prepared for ligation for 30 minutes, then heat kill for 15 minutes, and then digest my ligation product for 30 minutes. | ||
| + | *After digestion, I transformed T7-rbs-ThpA32 into a lefty. | ||
| + | *There were an abundance of colonies for rbs-B-rbs-C. I picked 5 colonies and grew them in culture tubes. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 15:48, 9 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *miniprepped 5 culture tubes (rbs-B-rbs-C). | ||
| + | *picked 5 colonies from T7-rbs-A and grew them in culture tubes. | ||
| + | *the sequences for rbs-ThpD-TT were all great. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 20:39, 10 August 2007 (EDT)</font>''' | ||
| + | *miniprepped 5 culture tubes for T7-rbs-A and sent them off to sequencing. | ||
| + | *set up digestion for rbs-B-rbs-C and rbs-D-TT. I digested rbs-B-rbs-C with BamHI and XhoI and rbs-D-TT with BglII and XhoI. | ||
| + | *I gel extracted the bigger band for rbs-B-rbs-C (3759bp), and I gel extracted the smaller band for rbs-D-TT (1058bp). I cleaned them up afterwards. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 14:23, 11 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | * I took my cleaned digested samples and ligated them (30mins), heat kill (15mins), digest the ligation product (30mins), and then transformed it into a righty (rbs-B-rbs-C-rbs-D-TT). | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 14:23, 13 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *Since Ricky grew 5 colonies into culture tubes for me yesterday, I came in and miniprepped my samples. | ||
| + | *Sequences came in for T7-rbs-A and everything seemed to match up perfectly. | ||
| + | *Seeing that Vinni's assay didn't work with the T7 promoter, I decided to use a pTet promoter without an rbs. I had to ligate an rbs to ThpA and then transform it into a righty. | ||
| + | *I transform pTet plasmid into a lefty. (70uL water, 10uL KCM, 1uL plasmid, 60uL competent cells) | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 14:23, 14 August 2007 (EDT)</font>''' | ||
| + | ---- | ||
| + | *sequences came back for rbs-B-rbs-C-rbs-D-TT and they matched up well. | ||
| + | *grew rbs-thpA into culture tubes (4) and pTet 1091. | ||
| + | |||
| + | '''<font color=lightblue> [[User:Nnguyen|Nnguyen]] 14:23, 15 August 2007 (EDT)</font>''' | ||
---- | ---- | ||
Latest revision as of 05:23, 16 August 2007
My Construction Files
My Sequencing Files
<font/><center/><b/>Master Plan</b></center>
Lefty Righty Lefty Righty Lefty Righty
Promoter pTet rbs-ThpA rbs-ThpB rbs-ThpC rbs-ThpD TT
Lefty Lefty Righty
pTet-rbs-ThpA rbs-ThpB-rbs-ThpC rbs-ThpD-TT
Lefty Righty
pTet-rbs-ThpA rbs-ThpB-rbs-ThpC-rbs-ThpD-TT
entire operon
pTet-rbs-ThpA-rbs-ThpB-rbs-ThpC-rbs-ThpD-TT
Nnguyen 15:40, 25 June 2007 (EDT)
Today I came in and I started my tutorial on internal sites because I missed a week of work last week. I completed the tutorial, and I was given my task for the week. Chris gave me a template to work on. The gene is called Streptomyces chrysomallus. There are four genes of interest in the template, and I had to make oligos for these genes. I had to construct two oligos for each gene, a forward primer and a reverse primer. In order to construct the oligos, I used the enzymes BglII and XhoI. BglII was used for the forward primer and XhoI was used for the reverse primer. In 2 out 4 genes, there were internal sites which meant that I had to make additional oligos.For gene ThpA, EcoRI was within it and so I had to make a primer that would remove it. The sequence for EcoRI was gaattc, and I changed it to gagttc. gag and gaa both code for the same amino acid which is glutamic acid. XhoI was within gene ThpB, and again I had to make a primver that would remove the restriction enzyme.
The sequence for XhoI is ctcgag, and I changed it to cttgag. ctc and ctt both code for the same amino acid which is leucine. In order to remove XhoI from ThpB, I construct a forward primer and a reverse primer. The reverse primer is the complimentary side of the forward primer. Compared to the oligos that I constructed for the beginning and end of each gene, the primers used to remove XhoI do not have restriction enzymes on them. Compared to ThpB, ThpA only has 2 primers and not 4 primers because the EcoRI was very close to the beginning of the gene. Therefore, I incorporated EcoRI into the forward primer for the gene ThpA. In the end, I ended up with 10 oligos.
At the end of the day, Sam showed the high school students how to pour agar into plates, and we left the plates on the counter throughout the night.
Nnguyen 15:40, 26 June 2007 (EDT)
I came into work today and helped the other high school students a little bit with their project while I was waiting for my oligos to arrive. They have not arrived yet; however, in the meantime, Sam and Arthur showed us how to make LB Agar and LB Broth. We poured 500mL of water into each glass bottle. For LB Agar, we added 20g of agar powder, and for LB Broth, we added 12.5g of broth powder. After adding the right amount into the 500mL of water, we shook the bottle until the powder dissolved. After that, we put all the glass bottles in a tray, untwisted the cap so that when they're put in the machine, they wouldn't break due to high pressure, and we added water and tape the caps. After we finished with the preparations, we put the tray into the autoclave machine for 25 minutes; however, it is better to let it sit in there for awhile to let it cool down. The agar that was placed in the autoclave machine did not work because the previous person who used it did not reset. We took out the agar bottles and let the machine exhaust for a while, and then we put it back in the autoclave for 25 minutes. After it finished, we let it stayed in there for a while so it can cool down. After taking them out, we added antibiotics to the agar bottles. We added AMP (antibiotics) and CAM (antibiotics) to 3 of the agar bottles, and we shook it. In the other agar bottles, we only added AMP. After shaking the bottles, we waited until the bubbles disappear and then we started pouring them into plates. If there were bubbles left in the plate, we put the flame directly on the agar so the bubbles go away. The plates that consist of AMP, we marked it with the color red. The plates that consist of AMP and CAM, we marked it with blue and red.
Nnguyen 23:57, 26 June 2007 (EDT)
At around 2:00, the oligos that I ordered yesterday arrived. I added water to each tube because the primers came in a form of powder. On each tube, it tells how much powder it is in nanomol. I multiplied the number of nanomol by 10 and that was how much water I added. For example, I added 385 microL of water into nn001.
nn001: 38.5 nmol nn002: 27.5 nmol nn003: 29.0 nmol nn004: 29.4 nmol nn005: 37.7 nmol nn006: 33.6 nmol nn007: 26.5 nmol nn008: 29.9 nmol nn009: 27.5 nmol nn010: 31.3 nmol
The DNA sequences for each of primers are:
nn001:cgtacAGATCTatgaccgccgcaccagcagattttgcccgtgcccgaagcgaGttcctcagtatcg nn002:cgcatctcgagtcaggtggcgaacgggccta nn003:ctagaAGATCTgtgaccatcaccccgcccgc nn004:cgtcactcgagtcaggccgtttcgcggacgc nn005:ggttcgagcggctcctTgaggaccagggctccggac nn006:gtccggagccctggtcctcAaggagccgctcgaacc nn007:ctagtAGATCTgtgatcgtccgatcgttcag nn008:gtctactcgagtcaggcctcctcggtcagca nn009:cggatAGATCTatgaccaccgaagtacgcgc nn010:gtctactcgagtcaggcctcctcggtcagca
After adding water, I vortex them so that all the loose particles would settle down, and the tubes were put on ice. After placing the tubes in ice, I started predicting my PCR product. My predictions are: ThpA=553 bp; ThpB=1285 bp; ThpC=421 bp; ThpD=916 bp.At the end of the day, I put all 10 tubes in the freezer (below 20).
Nnguyen 14:06, 27 June 2007 (EDT)
Today, I came in and found out that my template has not yet arrived. While I was updating my notebook, I realized that I have the same two primers (nn008 and nn010). nn008 is correct; however, nn010 is incorrect. I just finished putting my correct primer (nn011) on order.
The correct DNA sequence for nn010 is:
nn011:gtcagctcgagctacttcaccggggtgaagt
Without nn011, I am still able to continue with my work even if my template arrives today.
When my template arrived, the DNA was embedded in an organism and so I had to isolate the DNA. I used the Ultra Clean Mircobial DNA isolation Kit to isolate the DNA. After isolating the DNA i was ready for PCR. First, I had to dilute the primer and so I added 1uL of primer to a new tube and I added 9uL of water to each tube. After making PCR tubes and putting the right amount of primers (forward, reverse), template, enzyme, buffer, dNtps and water, I put my PCR tubes in the PCR machine. I used the 3-step program and the process took 1 hour and 14 minutes.
I attempted to PCR again. I had 6 PCR tubes: 3 PCR tubes containing the enzyme expand and Dmso 100% and the other 3 PCR tubes contained the enzyme phusion and no Dmso 100%.
Nnguyen 16:14, 28 June 2007 (EDT)
I checked on my PCR tubes today and found out that the PCR tubes that contain phusion did not work. The PCR tubes that expand worked. This time, ThpA came out right; however, ThpB did not come correctly.
I also cleaned up ThpA and ThpC and digested them. After putting them in the incubator for 2 hours, I took them out and cleaned them up again. After digestion, I underwent ligation by adding vector 9145, my insert (ThpA and ThpC), ligase buffer, ligase and water. After that, I let the tubes sit for 30 minutes to an hour.
Note: Ligation is occuring in two different tubes (ThpA and ThpC)
After ligation was finished, I carried out transformation. I needed 220uL of competent cells and salt solution ( 30uL of KCM and 50uL H2O). After added the salt solution to the competent cells, I added 100uL of the solution to each ligation tube. I mixed it and put them on ice for 10 minutes. After that, I heat shock if for 90 seconds in 42C. I put it back on ice for 2 minutes. I added 110uL to an agar dish, and I used the beads to spread the solution. In the end, I put the agar dishes in the incubator for 16 hours.
The correct reverse primer for ThpD came and I added water to the tube. After vortexing it, I took out 1uL of primer and added to a 9uL of water which makes a total of 10uL. After adding the correct primers, template, enzyme, buffer, dNtps, and water, I placed my PCR tubes into the PCR machine. Unfortunately, PCR did not work for ThpD.
Nnguyen 19:21, 29 June 2007 (EDT)
I came in today hoping that I can get ThpD to work. I tried three different techniques hoping that PCR would work. The first PCR tube, I used the enzyme phusion and HF buffer 5x and everything else is the same as the previous PCR tubes. The second PCR tube, I used the enzyme phusion and GC buffer 5x. The third PCR tube, I used the enzyme expand and expand buffer 10x. After taking the tubes out of the PCR and gelling it, the tube with expand did not have a band; the tube with phusion and GC buffer had too many bands, and the tube with phusion and HF buffer showed a satisfying band.
Since PCR did not work when I tried to sew together the two parts in ThpB, I decided to gel extract the 2 parts. I ran 60uL of ThpBF/ThpBIR and 60uL of ThpBR/ThpBIF on the gel and the bands did not show up at all, even the marker. With the remaining 20uL of ThpBF/ThpBIR and 20uL of ThpBF/ThpBIR, I gelled that and there were faint bands for them. After cutting out the piece of gel, I added 500uL of ADB buffer and heat up the tube so the gel can melt. After it melted, I usedthe DNA Recovery Kit to clean up my gel. After clean-up, I made a solution with Dmso 100% so I can carry out my second PCR.
I finished cloning ThpA and ThpC in the agar dishes. Now I am growing them in LB broth. I have 7 LB broth tubes: 5 from ThpA and 2 from ThpC. I pipeted out 35mL of LB Broth and put 5mL in each tube. I labeled each tube ThpA1, ThpA2.....ThpA5, ThpC1, and ThpC2. Before doing anything to these tubes, I need to make a master solution for my colony PCR.
For Colony PCR I need:
21.8 uL water 2.5 uL buffer .5 dNTPs .1 uL Taq .5 uL Forward primer .5 uL reverse primer
Total Volume: 25.9 uL
Note: the primers are G0001 and Ca998
I multiply this solution by 8 because I need to divide the solutions into 7 different tubes and by multiplying it by 8 allows me to have extra if I needed it. The total volume after multiplying by 8 is 207.2 uL. After making my master solution, I put 25.9 uL in each PCR (7) tubes (the labels should correspond to the LB Broth tubes). I took a pipet tip and dipped it in a chosen colony, twirl it in the corresponded PCR tube, and then drop the tip into the corresponded LB Broth tube.
Goals for Saturday, June 30,2007
1. gel ThpB (PCR #2)
2. PCR clean-up ThpD and ThpB (if PCR works)
3. Digestion, clean-up ThpD and ThpB (if PCR works)
4. Ligation-->ThpD and ThpB (if PCR works)
5. Transformation
6. Colony PCR-->gel, miniprep
Nnguyen 15:24, 30 June 2007 (EDT)
I came in this morning and retrieved my Colony PCR tubes (7) upstairs and my PCR tubes (ThpB PCR #2). I put 5uL of each in a differnt tubes and put .5 uL of buffer in them, and I put them on gel. ThpB turned out satisfying.
Both of the bands for ThpC1 and ThpC2 were satisfying. Choosing only 3 our of 5 ThpA colony PCR tubes, and both of ThpC colony PCR tubes, I miniprep them. Before I miniprep, I must pour some liquid media in a small blue tube, but I should leave some in the LB Broth tube for later use. After pouring them in the blue tubes, I centrifuge the 5 tubes for 6 minutes to suspend the pellets. After that, I followed the miniprep protocol. After I finished with miniprep, I am supposed to send it to sequencing; however, I will do that on Monday.
Since ThpB is good to go, I PCR clean-up ThpB and ThpD. After cleaning up, they were ready for digestion. After preparing the solution for digestion, I placed the tubes in the incubator for 2 hours. After taking it out from the incubator, I cleaned up again. However, this time when I elute the solution, I only added 10uL of water rather than 80uL. After cleaning up, I went on and prepared a solution for ligation. After adding water, insert, vector, ligase buffer, and ligase, I centrifuge the tubes and let them sit for 30 minutes to an hour.
After ligation was completed, transformation can take place. I need 220uL of competent cells, and I also need a salt solution (30uL of KCM and 50uL of H2O). I added the salt solution to the competent cells and distributed 100uL into each ligation tubes (2). I mixed the tubes and put them on ice for 10 minutes. After taking them off the ice, I heat shock the tubes for 90 seconds in 42C. I put them on ice for 2 minutes and I added the entire solution (110uL) onto the agar dish (there are two: ThpB/9145 and ThpD/9145). I used the beads to spread the solution throughout the plate. After finishing, I put the two agar plates into the incubator for 16 hours.
Note: When I am transferring the solutions into an agar dish, I must be in a sterile area.
My goals for today were all accomplished!!!
Nnguyen 00:15, 2 July 2007 (EDT)
I finished my construction files for all my genes today.
After I minipreped ThpA and ThpC, I sent them off for sequencing. I loaded 4uL of plasmid into a different eppendorf tube and added 4uL of water. I sent off 5 tubes for sequencing. Since ThpA and ThpC are both less than 700bp, I only needed one primer (ca998) rather than two.
I prepared a master solution for colony PCR for ThpB and ThpD. I picked 4 colonies for ThpB and ThpD. Altogether, I have 8 PCR tubes, and I also grew them in LB Broth AMP. I put the LB Broth tubes in the shaker for 16 hours.
Nnguyen 17:19, 3 July 2007 (EDT)
The sequences for ThpA2, ThpA3, ThpA4, ThpC1, and ThpC2 were sent to me. I checked them and Richard said that ThpA2 is contaminated, and ThpA4 either has multiple inserts or none. ThpA3, ThpC1, and ThpC2 worked fine. However, when I checked the sequences to see whether if they matched, ThpA3 did not match very well. ThpA3 had the restriction sites (BglII and XhoI) and about the first 20 base pairs of the gene. It also has about the last 20 base pairs of the gene. Everything in between was junk. In the end, ThpA3 did not work out. ThpC1 and ThpC2 matched up very well; however, in both of the sequences, there was one mutation. They weren't silent mutations. For ThpC1, the mutation took place at 182 bp (only gene ThpC was examined). The desired codon was valine (GTG), but what I got was alanine(GCG). For ThpC2, the mutation took place at 25bp (only gene ThpC was examined). The desired codon was isoleucine(ATC) but I got valine (GTC).
I decided to redo ThpC. I prepared a solution for ThpC and will place it in the PCR machine today before I leave.
As for ThpA, I decided to pick 5 (ThpA1a, ThpA2a....ThpA5a)new colonies to grow in broth, and I colony PCR them today.
Nnguyen 00:08, 5 July 2007 (EDT)
Before leaving on Tuesday, I put a PCR tube into the PCR machine. I redid ThpC, hoping that there would be no mutation this time because it is believed that in the previous one, the mutation occurred the PCR. After PCR clean-up, I sent a sample to Quintara hoping that the sequence matches up with what I predicted. Before sending it to Quintara, I had to dilute my primers twice and then add 8uL of the primer into a different tube, and 5uL of the PCR product. I sent Quintara two samples, one for each primer. Besides sending a sample from ThpCb to Quintara, I also sent another sample from ThpA. The samples I sent from ThpA was from the PCR product I did a while back.
Richard sent me the sequences for ThpB and ThpD. Looking through 3 samples of ThpB, one matched up very well; however, there were a couple of mutations. Looking through 3 samples of ThpD, one matched up perfectly, so that one was good. Since I only used the enzyme phusion for ThpD, I decided to make new PCR tubes that consist of phusion for ThpA, ThpC and ThpB.
After cleaning up ThpCb (the one that I sent to Quintara today), ThpCb went through digestion, clean-up, ligation and then transformation.
I chose 10 more colonies from my plate from ThpB/9145. I was unable to grow them in culture tubes because the tubes weren't available during the time. This time, I twirled the colony in the PCR tube and then marked an "X" on a different plate.
Nnguyen 18:28, 6 July 2007 (EDT)
I came in today and I retrieved my PCR colony tubes (10 from ThpB) and my PCR tubes (ThpBc, ThpBIc). After gelling them, out of the 10 PCR colony tubes 2 had satisfying bands. ThpBc and ThpBIc had satisfying bands. After seeing that the bands were good, I gelled the entire volume of ThpBc and ThpBIc. After gelling them , I gel extracted them and cleaned them up. After cleaning, ThpBc was ready for its second PCR (sewing). Instead of using expand, I used phusion with the addition of Dmso 100%. There are two tubes for PCR #2 because while I was gel extracting, there were two bands for ThpBc, and I extracted both of them because they were within the range that I wanted. After PCR was done, I gelled and there weren't any bands. I tried sewing once more;however, this time I used expand, and it still didn't work.
For ThpCb and ThpA, I picked 8 colonies from each plate and grew them in culture tubes and colony PCR them. After Colony PCR, I ran them on gel and there were bands and ThpCb seemed to have the desired band. After choosing 3 samples for ThpCb (ThpCb3,ThpCb6,ThpCb8) and ThpA(ThpA3b, ThpA6b, ThpA8b), I miniprepped along with 2 samples from ThpB.
Nnguyen 01:40, 9 July 2007 (EDT)
I sent ThpCb3, ThpCb6, ThpCb8; ThpA3b, ThpA6b, ThpA8b, ThpB4a, ThpB5a to sequencing. For ThpB, I needed to add the ca998 and G01001 because it has 1200 base pairs.
For ThpBc, I tried sewing the two parts together. However, this time I only have one tube because I did not have enough of the part with the internal site.
Nnguyen 15:35, 10 July 2007 (EDT)
Today, Richard sent me the sequences for the samples that I sent yesterday. Fortunatley, 2 of the ThpCb (ThpCb3 and ThpCb6) samples worked. For the other samples, there were a couple of mutations.
I sent in 10 more sample for ThpA because the sequences from the PCR product for ThpA was fine.
I transformed the right RBS I got from Vinni into a lefty. I also transform ThpCb6 and ThpD2 into righty cells.
Clone Savor: I took 10uL the broth media that I had of ThpCb6 and ThpD2 pipetted unto a piece of paper (clone saver.
Nnguyen 18:43, 11 July 2007 (EDT)
The sequences for ThpA came in and one out of 10 matched very well; unfortunately, there was one mutatioin.
The PCR product for ThpB has two mutations. The mutations might be within the genome itself or it might be a result from PCR.
I decided to send ThpB back to sequencing. This time, before sending it to sequencing, I'm going to put it in the PCR machine. I used the BglII and XhoI primers instead of using the primers that will remove the internal sites.
Transformation worked great for ThpCb6, ThpD2, and RBS. I picked out a colony and grew that in liquid media.
Lefty: RBS
Righty: ThpCb6, ThpD2
I picked out 8 more colonies from plate ThpA/9145 and colony PCR them. Hopefully after miniprepping them and sending them to sequencing, the sequences will turn on out great.
Nnguyen 12:38, 12 July 2007 (EDT)
I came in and took out the liquid media for ThpCb6, ThpD2, and RBS from the incubator. I minipreppred those and got them ready for digestion. For RBS, I had:
20uL of water 6 uL of plasmid 3uL 10x buffer NEBuffer #2 .5uL of BamHI .5uL of XhoI.
For ThpCb6 and ThpD, everything is the same as RBS except for the enzymees that I used. I used BglII and XhoI for ThpCb6 and ThpD2. The total volume for my digestion tubes was 30uL. The tubes were put in the incubator for 2 hours. After digestion, I prepared the tubes for ligation. The duration for ligation was 30 minutes to an hour. In the ligtaion tubes, I had:
4uL water 3uL insert (smaller piece-->ThpCb6,ThpD2) 1uL vesctor (larger piece-->RBS) 1uL ligase buffer 1uL ligase
The total volume for ligation wa 10uL.
After ligation was finished, I had to heat kill the ligase for 15-20 minutes. After heat kill, I digested the ligation product with BamHI and BglII. The volume for the digested ligation tubes was 20uL. In the tubes I had:
10uL ligation product 7uL water 2uL buffer #2 .5uL BamHI .5uL BglII
After digestion, I got ready for transfomation. Instead of using competent cells, I used the lefty and righty cells. I had to grow RBS-ThpCb6 into a lefty strain, and I grew RBS-ThpD2 into a righty strain. I put the dishes into the incubator for 16 hours.
ThpA has been having some problems with sequencing because the sequences haven't been matching up very well. In order to fix this problem, I carried out the process of ligation again for ThpA.
Nnguyen 13:00, 13 July 2007 (EDT)
I came in today and retrieved my plates and found out that there were good colonies on RBS-ThpCb6, RBS-ThpD2, and ThpA.
The sequences for ThpB came in today, and it seems that the two constant mutations are in the template itself. So now, ThpB is ready to go. Two days ago, I transformed ThpB5a into a righty strain. I grew one colony in liquid media. Today, I miniprepped it and carried out with digestion for 2 hours. I gel extracted the band that I wanted, and then I cleaned-up. After cleaning up, I carried out the process of ligation (the solution consists of the same substances as the previous ligations on July 12). The total volume was 10uL. After ligation, I heat kill the ligase for 15 minutes. I digested the ligation product (the solution consists of the sambe substanes as the previous digestion of the ligation product as the previous day, July 12). After digesting for 30 minutes-1 hour, I carried out the process of transformation. I transformed RBS-ThpB5a into a righty strain. I left the plate in the incubator for 16 hours.
Since there were good colonies for ThpA, I picked 10 colonies and PCR colony them, and I also grew them in culture tubes for 16 hours in the incubator.
Nnguyen 13:06, 14 July 2007 (EDT)
I came in today and retrieved my plate from the incubator. I choose 5 colonies and put them in culture tubes. I put the culture tubes in the incubator for 16 hours.
I gelled the Colony PCR products for ThpA and found out that 4 (3,4,5,9 worked) out of 10 colonies had the desired bands. I miniprepped those along with 10 samples for ThpCb6 (5 samples) and ThpD2 (5 samples).
Nnguyen 15:29, 16 July 2007 (EDT)
I retrieved my culture tubes and miniprepped them (5 tubes of ThpB5a).
I sent RBS-ThpCb6-4(lefty), RBS-ThpD2-5 (righty), RBS-ThpB5a3 (righty) to sequencing today. i also sent the 4 samples of ThpA to sequencing today.
I prepared to ligate my composite parts for ThpC and ThpD today; however, I am going to stop after the first digestion because I have to leave early today.
For RBS-ThpCb6, I will use BamHI an XhoI. By using BamHI and XhoI, BglII will get methylated. After digestion is completed, I will gel it and cut out the larger band. For RBS-ThpD2, I will use BglII and XhoI. By using BglII and XhoI, BamHI will get methylated. After digestion is completed, I will gl it and cut out the smaller band. After cutting it, I will clean up my digestion product.
Nnguyen 12:50, 17 July 2007 (EDT)
Since RBS-ThpCb64 and RBS-ThpD25 didn't work after digestion. I'm planned to try another digestion and send that to sequencing to see whether if it matches up with what I want. I digested RBS-ThpCb61 and RBS-ThpD22. The predicted band for RBS-ThpCb61 is 2468bp, and the predicted band for RBS-ThpD22 is 900 bp. After gelling it, the band for RBS-ThpCb61 seems as if it was closer to 2000bp rather than 2400bp. There was only one band for RBS-ThpD22. There were supposed to be two bands because I was supposed to extract the smaller band.
Seeing that the digestions aren't working, I choose 10 more colonies from each dish and colony PCR them as well as growing them in broth. I gelled the colony PCR products and ThpCb6 had a band but it was a lot smaller than what was expected. ThpD2 did not have any band at all.
The sequences arrived from Quintara and in all the samples for ThpA, there was at least one point mutation. In conclusion, John and I decided that we should try a quickchange and change the guanine to an adenine (this happened in sample ThpA3). The amino acid that I have is glycine(GGG) and the desired amino acid is glutamic acid (GAG). I order two new primers yesterday. The forward primer is 51 base pairs (25bp before the adenine and 25bp after adenine). The reverse primer is the reverse complimentary of the forward primer.
Nnguyen 13:02, 18 July 2007 (EDT)
I came in today and miniprepped 2 samples of ThpCb6 (RBS-ThpCb67a, RBS-ThpCb69a) and ThpD2(RBS-ThpD23a, RBS-ThpD26a. These are the samples I colony PCR yesterday. I also miniprepped 8 samples of ots AB (lefty) for Vinni.
I checked my sequences for RBS-ThpCb64 and RBS-ThpD25 and they matched up perfectly. I am going to try to redigest using the same plasmids since the sequences matched up perfectly. After digestion was finished, I gelled it and I extracted the desired bands. For ThpCb, I extracted the larger band (2400) and for ThpD2, I extracted the smaller band (900). The larger band for ThpD2 was about 2000. After gel extraction, I cleaned it. After cleaning it, I ligated the two composite parts together. I heat kill for 15 minutes afterwards and then transformation. I transformed the composite parts into righty.
At the end of the day, I realized I didn't send all of my RBS-ThpB5a samples to sequencing and so I prepared tubes for sequencing. I sent 4 different samples (ThpB5a1,2,4,5).
Nnguyen 14:46, 20 July 2007 (EDT)
The sequences came back for RBS-ThpB5, and it seems as if all 4 samples have the the same mutation (an additional mutation). We decided to send one of the sequences for RBS-ThpB5a to campus so they can sequence it.
3.2 uL primer 5uL plasmid 4.8uL water Total Volume: 13uL
- The oligos I ordered for ThpA3 came.
NN012 (forward): tcgatgcgccacgagtggaggacggagccgcgatctggcgcatagcccgcg NN013(reverse): cgcgggctatgcgccagatcgcggctccgtcctccactcgtggcgcatcga
<b/>Quickchange</b>
1. set up 2 separate primer extension reactions
5uL 10x Pfu buffer 1uL 10um primer .1-.2 uL plasmid template 1uL 10uM dNTP 1uL Pfu turbo polymerase 41.8uL water Total Volume: 50uL
2. Incubate:
1. 94 degrees: 30secs 2. 95 degrees: 30secs 3. 55 degrees: 1 min ( I changed this to 1:30 minutes) 4. 68 degrees, 2 min/kb (I changed this to 5 minutes)
- I repeated steps 2-4 three times and held it at 4 degrees. Total cycles: 4
3. Combine 25uL from each tubes into one tube and add 1uL Pfu polymerase. Incubate (look at previous step) for 18 cycles.
4. Remove 25uL of the reaction, add 10 units (.5uL) of DpnI enzyme. Mix and incubate at 37 degrees for one hour.
5. Transform 1uL of reaction into competent cells and plate them. ( I transformed it into lefty cells and then plated them).
- colony PCR RBS-ThpCb64-RBS-ThpD25, gelled them and miniprepped samples 7,8,9, and 10.
Nnguyen 14:46, 21 July 2007 (EDT)
I came in today and colony PCR. I had 3 colonies after transforming my quickchange into a lefty. While preparing for colony PCR, I grew them in liquid media as well.
Nnguyen 15:21, 23 July 2007 (EDT)
I came in and retrieved my culture tubes, and Vinni helped me miniprepped them. I sent those off the Quintara (thpA-->quickchange) While I was making my Ape files, I realized that my genes didnt have a BamHI site and so we had to figure out a way to insert it in.
- I made 5 oligos (common primer--> have XhoI and 20 bp from plasmid with BamHI site in front of XhoI, and the other 4 oligos will have 20bp from the end of the gene with BamHI right after it--> note: i reverse the oligios)
nn016:gtcagggatcctaaCTCGAGctgcagttgcaggcttcctc (common primer) nn017:gtcagggatcctcaggtggcgaacgggccta (reverse primer for thpA) nn018:gtcgaggatcctcaggccgtttcgcggacgc (reverse primer for thpB) nn019:gtcgaggatcctcaggcctcctcggtcagca (reverse primer for thpC) nn020:gctgaggatccctacttcaccggggtgaagt (reverse primer for thpD)
- I also order 2 more primers for RBS-ThpB so when I send it off to sequencing, the sequence would show the 3rd mutation.
nn014:ctccggactcaacaagcccg (forward primer) nn015:gccgtagccgctgatggact (reverse primer)
Nnguyen 15:21, 24 July 2007 (EDT)
The sequences for ThpA (quickchange) arrived. Out of 3 samples, one sample worked.
When the primers came in today, I dilute it to 10uM and then prepared for PCR.
41 uL water 5 uL buffer 10x 1 uL Reverse primer (it varies-->depends on the plasmid that I used) 1 uL dNTPS .5 plasmid (it varies because I'm using 4 different genes) .5 expand Total Volume: 50 uL
Nnguyen 15:21, 25 July 2007 (EDT)
After retrieving my PCR tubes, I gelled them and found out that only ThpCb6 and RBS-ThpCb64 worked. The other samples did not have any bands.
- I tried PCR once more but this time I elongated the time to 3 minutes and changed the temperature to 50C.
- Once again, PCR did not work. I tried PCR once more but this time we decided to have two different sets: one with Dmso 100% and one without Dmso 100%. In both sets, I doubled the amount of oligos. Each tubes consists of (there are six different tubes for each set:ThpA3, ThpB5a, ThpC6,ThpD2, rbs-ThpB5a3, rbs-ThpD25):
<u/>Set #1: With Dmso 100%</u> <u/>Set #2: Without Dmso 100%</u>
34uL water 39uL water
5uL 10x buffer 5uL 10x buffer
5uL Dmso 100% 2uL common primer
2uL common primer 2uL reverse primer(varies)
2uL reverse primer(varies) 1uL dNTPs
1uL dNTPs .5uL plasmid (varies)
.5uL plasmid(varies) .5uL expand
.5 expand
Total Volume: 50uL
Since ThpCb6 and rbs-ThpCb64 worked, I cleaned the PCR product and prepared my tubes for digestion.
20uL water 6uL PCR product 3uL buffer #2 .5uL BamHI .5uL DpnI Total Volume: 30uL
- After digestion, I gel extracted them, cleaned them, and then prepared for ligation.
4uL water 4uL digested PCR product 1uL ligase 1uL ligase buffer Total Volume: 10uL
- After ligation was transformation. I transformed ThpCb6 into competent cells and rbs-ThpCb64 into lefty.
Nnguyen 15:21, 26 July 2007 (EDT)
I came in and retrieved my PCR products and gelled them. The samples that consist of Dmso 100% had a bright band except for ThpD2 and rbs-ThpD25. I digested ThpA3, rbs-ThpB, ThpB, rbs-ThpD25, ThpD2. I digested the samples with Dmso 100% except for ThpDs. I gelled the digestion to gel extract; however, there weren't any bands. Due to a problem with the incubator (the temperature was beyond what it was supposed to be), I believe that was the cause of my outcome. I will start all over tomorrow.
- I colony PCRed 5 colonies from each of the plate that I transformed yesterday (ThpCb6, rbs-ThpCb64-->lefty, rbs-ThpB5a3-->righty (without BamHI)). I also grew them in liquid media. I gelled my colony PCR products and there were bands for all the samples. rbs-ThpB5a3 seemed to have the right band; however, the others didn't.
Nnguyen 15:21, 27 July 2007 (EDT)
- I miniprepped all of rbs-thpB5a3 (without BamHI) and rbs-ThpCb64-3, rbs-ThpCb64-5, ThpCb6-3, and ThpCb6-5.
- I gel extracted ThpA3 and ThpB5a and rbs-ThpB5a3 {all with BamHI) and then cleaned them up, ligated them, and then transformed them. ThpA3-->Righty, ThpB5a-->Lefty, rbs-ThpB5a3-->Lefty.
- I also transformed rbs-ThpCb645 and rbs-ThpCb643 into righty competent cells.
Nnguyen 15:21, 28 July 2007 (EDT)
- There were colonies on only rbs-ThpCb645,3. There were one colony for thpA3 and ThpB5a.
- Colony PCRed them and grew them in culture tubes (8-->3 for each rbs-C and one for A and one for B)
Nnguyen 15:21, 20 July 2007 (EDT)
- Colony PCR was quite a success.
- miniprepped ThpA3,ThpB5a, rbs-ThpCb645-1,2 and rbs-ThpCb643-1,2.
I PCRed rbs-ThpD2-5 and ThpD2 once more. I made four tubes, one without Dmso 100% and one with Dmso 100%.
<u/>Tube 1 with Dmso 100%:</u> <u/>Tube 2 without Dmso 100%:</u>
34uL water 39uL water
5uL 10x buffer 5uL 10x buffer
5uL Dmso 100% 2uL reverse primers (nn020)
2uL reverse primer (nn020) 2uL forward primer (ca998)
2uL forward primer (ca998) 1uL dNTPs
1uL dNTPs] .5uL template (rbs-ThpD2-5/ThpD2)
.5uL template (rbs-ThpD2-5/ThpD2) .5uL expand
.5uL expand
Total Volume: 50uL
- Test Digestion for ThpA3 and ThpB5a:
6uL water 2uL plasmid (ThpA3/ThpB5a) .5uL BamHI .5uL BglII 1uL buffer #2 Total Volume: 10uL
- Gelled ThpD2, rbs-ThpD2-5, ThpA3 and ThpB5a. There were bands for A and B but there weren't anything for ThpD2 and rbs-ThpD2-5.
- PCR rbs-ThpD2-5 again. This time without Dmso 100% (Farnaz did one tube and I did another).[same contents as previous PCR tubes]
Nnguyen 15:48, 31 July 2007 (EDT)
- Ligated ThpA3 and ThpB5a with BamHI again and then transformed them.
- PCR rbs-ThpD2-5 again. This time we used phusion rather than expand. There are a total of 4 PCR tubes.
<u/>Set 1 (with Dmso 100%):</u>
29uL water 29uL water
10uL buffer 5x(HF) 10uL buffer 5x(HF)
5uL Dmso 100% 5uL Dmso100%
2uL reverse primer (nn020) 2uL reverse primer (nn020)
2uL forward primer (ca998) 2uL common primer (nn016)
1uL dNTPs 1uL dNTPs
.5uL template (rbs-ThpD2-5) .5uL template (rbs-ThpD2-5)
.5uL phusion .5uL phusion
Total Volume: 50uL
<u/>Set 2 (without Dmso100%)</u>
34uL water
10uL buffer 5x (HF)
2uL reverse primer (nn020)
2uL forward primer/common primer
1uL dNTPs
.5uL template (rbs-ThpD2-5)
.5uL phusion
Total Volume: 50uL
- Gelled the PCR products and all them have bands except for rbs-ThpD2-5 without Dmso100% with the forward primer.
- Cleaned rbs-ThpD2-5 with Dmso100% with the common primer.
- Overnight digestion:
24uL water 20uL cleaned up PCR product (rbs-ThpD2-5) 1uL BamHI 5uL BamHI buffer Total Volume: 50uL
Nnguyen 15:48, 1 August 2007 (EDT)
- Gelled overnight digestion (rbs-ThpD2-5). Gel extracted the top utmost band, cleaned up, and then ligated. After ligation, I transformed rbs-ThpD2-5 into lefty.
- I colony PCRed 7 colonies from the righty-ThpA3 plate and one colony on the lefty ThpB5a plate. I gelled them and there weren't any bands.
Nnguyen 15:48, 2 August 2007 (EDT)
- Lefty rbs-ThpD2-5 has colonies! I grew 8 colonies in culture tubes.
- Miniprepped 4 our of 7 samples for ThpA3(ThpA3-3,5,6,7) and one for ThpB5a. I sent those samples to sequencing.
- I test digestd my miniprepped samples for two hours and then gelled them. There were bands, however, the bands did not seem right. I digested one more time; however, this time I digested with only BglI, and I used Buffer #3. I also digested rbs-ThpB5a3 along with ThpA3 and ThpB5a.
11.5uL water 6uL clean PCR 2uL 10x Nebuffer 3 .5uL BglI Total Volume: 20uL
When digestion was finished, I gelled the samples. Along the samples, I also gelled the clean PCR product to compare the differences. Unfortunately, there were only bands for the clean PCR product and not the digestion.
- The sequences arrived for the PCR product for ThpA3 and rbs-ThpB5a3 and rbs-ThpD2-5. The only sequence that seemed to work was rbs-ThpD2-5.
- Since phusion worked for rbs-ThpD2-5, I tried to use phusion for ThpA3, ThpB5a, and rbs-ThpB5a3 instead of expand.
29uL water 10uL buffer 10x (HF) 5uL Dmso 100% 2uL specific reverse primer Program used on PCR machine: 2uL common primer 3K; temperature: 55; elongation time: 3 minutes 1uL dNTPs .5uL template (varies) .5uL phusion Total volume: 50 uL
- I ordered the correct reverse primers for ThpA3 and ThpB5a. I'm planning to start from the beginning for these two genes unless the PCR tubes with phusion work.
Nnguyen 15:48, 3 August 2007 (EDT)
- Gelled PCR product from yesterday and they all had good bands. I cleaned them and prepared for digestion (2hrs)
- I gelled my digestions and gel extracted them, cleaned them, and prepared for ligation (30mins)
- After ligation, I transformed them. ThpA3-->righty, ThpB5a-->lefty, rbs-ThpB5a3-->lefty
I sent PCR products for ThpA3, ThpB5a, rbs-ThpB5a3 to sequencing (6uL water, 2uL PCR product).
- Ricky miniprepped 8 culture tubes for me for rbs-thpD25 and I sent them all off to sequencing.
Nnguyen 15:48, 4 August 2007 (EDT)
- The sequences came back for rbs-ThpD25, and fortunately, all of the sequences matched up very well.
- The PCR products for ThpA3 matched up very well but the BamHI and XhoI sites didn't match up.As for tbs-ThpB5a3 and ThpB5a, it had the same problem as ThpA3 but everything matched up.
- There were colonies on the plates I transformed yesterday. I grew 5 colonies in culture tubes.
Nnguyen 15:48, 6 August 2007 (EDT)
- miniprepped ThpA3, ThpB5a,rbs-ThpB5a3 (15 total-->5 from each plate)
- after miniprepping, I test digested (2hrs) all my miniprepped samples.
6uL water 2uL plasmid 1uL buffer 2 .5uL BamHI .5uL BglII Total Volume: 10uL
- I digested rbs-ThpD254 with BamHI and EcoRI, and I digested TT with BglII and EcoRI.
<u/>rbs-ThpD254</u> <u/> TT</u>
20uL water 20uL water
6uL plasmid 6uL plasmid
3uL 10x buffer 2 3uL 10x buffer 2
.5uL BamHI .5uL BglII
.5uL EcoRI .5uL EcoRI
Total Volume: 30uL
- I gel extracted the smaller band for rbs-ThpD254(~923bp), and I gel extracted the larger band for TT. I cleaned up and prepared for ligation (30min-1hr). rbs-ThpD254 is the insert and I put in 3uL. TT is the vector and I put in 1uL.
- After ligation, I heat kill for 15-20minutes and then I digested the ligation product. After digestion, I transformed rbs-ThpD254 into a righty.
- I gelled my test digest and there were bands for all of them but there were desired bands for certain samples so I picked those and send them off to sequencing (ThpA31,ThpA32,ThpA35,ThpB5a1,ThpB5a3,rbs-ThpB5a31,rbs-ThpB5a33 and rbs-ThpB5a35).
Nnguyen 15:48, 7 August 2007 (EDT)
- The sequences came for ThpA3, ThpB5a, and rbs-ThpB5a3 and they all seemed to matched up well.
- I prepared digestion for ThpA3, T7-rbs, rbs-ThpB5a35, and rbs-ThpCb6432.
- I digested ThpA3 with BamHI and BglII, T7-rbs with XhoI and BamHI, rbs-ThpB5a35 with BamHI and XhoI, and rbs-ThpCb6432 with BglII and XhoI.
- I would need to extract the smaller band for ThpA3 (537bp), the larger band for T7-rbs (2106bp), the larger band for rbs-ThpB5a35 (3340bp), and the smaller band for rbs0ThpCb6432 (428bp).
- Unfortunately when I gelled the digestions, there were bands; however, only some of them were bands that I wanted. I decided to re-digest them.
- After gelling the digestions for the second time, I got them same results. This time, I gel extracted T7-rbs, rbs-ThpB5a35 and rbs-ThpCb6432. ThpA3 had the larger band but it didn't have the smaller band.
- I cleaned up the ones that I gel extracted and prepared for ligation for rbs-B and rbs-C. The vector was rbs-B and the insert was rbs-C. I heat kill afterwards for 15 mins, and then digested the ligation product with BamHI and BglII. I transformed rbs-B-rbs-C into a lefty.
- Before I left, I set an overnight digestion and a test digestion for ThpA3 hoping that it would work.
<u/>Overnight Digestion:</u> <u/>Test Digestion:</u> 31uL water 6uL water 12uL plasmid 2uL plasmid 5uL buffer 2 1uL buffer 2 1uL BamHI .5uL EcoRI 1uL BglII .5uL XhoI Total Volume: 50uL Total Volume: 10uL
Nnguyen 15:48, 8 August 2007 (EDT)
- miniprepped 5 culture tubes for rbs-ThpD254-TT.
- Gelled overnight digestion and test digestion. There was only one band for all overnight digestion, and there were two bands for the test digestion.
- Realizing that I had to digest ThpA3 with BglII and XhoI instead of BglII and BamHI, I redid the digestion with the correct enzymes. I then extracted the smaller band which is ~546bp and then I cleaned up the product.
- After clean up, I prepared for ligation for 30 minutes, then heat kill for 15 minutes, and then digest my ligation product for 30 minutes.
- After digestion, I transformed T7-rbs-ThpA32 into a lefty.
- There were an abundance of colonies for rbs-B-rbs-C. I picked 5 colonies and grew them in culture tubes.
Nnguyen 15:48, 9 August 2007 (EDT)
- miniprepped 5 culture tubes (rbs-B-rbs-C).
- picked 5 colonies from T7-rbs-A and grew them in culture tubes.
- the sequences for rbs-ThpD-TT were all great.
Nnguyen 20:39, 10 August 2007 (EDT)
- miniprepped 5 culture tubes for T7-rbs-A and sent them off to sequencing.
- set up digestion for rbs-B-rbs-C and rbs-D-TT. I digested rbs-B-rbs-C with BamHI and XhoI and rbs-D-TT with BglII and XhoI.
- I gel extracted the bigger band for rbs-B-rbs-C (3759bp), and I gel extracted the smaller band for rbs-D-TT (1058bp). I cleaned them up afterwards.
Nnguyen 14:23, 11 August 2007 (EDT)
- I took my cleaned digested samples and ligated them (30mins), heat kill (15mins), digest the ligation product (30mins), and then transformed it into a righty (rbs-B-rbs-C-rbs-D-TT).
Nnguyen 14:23, 13 August 2007 (EDT)
- Since Ricky grew 5 colonies into culture tubes for me yesterday, I came in and miniprepped my samples.
- Sequences came in for T7-rbs-A and everything seemed to match up perfectly.
- Seeing that Vinni's assay didn't work with the T7 promoter, I decided to use a pTet promoter without an rbs. I had to ligate an rbs to ThpA and then transform it into a righty.
- I transform pTet plasmid into a lefty. (70uL water, 10uL KCM, 1uL plasmid, 60uL competent cells)
Nnguyen 14:23, 14 August 2007 (EDT)
- sequences came back for rbs-B-rbs-C-rbs-D-TT and they matched up well.
- grew rbs-thpA into culture tubes (4) and pTet 1091.
Nnguyen 14:23, 15 August 2007 (EDT)