Paris/July 12
From 2007.igem.org
(→Acinetobacter microscopy) |
(→E.coli pKS::DGAT on LB oletate medium) |
||
| Line 41: | Line 41: | ||
== E.coli pKS::DGAT on LB oletate medium == | == E.coli pKS::DGAT on LB oletate medium == | ||
| - | We incubated E.coli pKS::DGAT on different LB medium (see [[Paris/ | + | We incubated E.coli pKS::DGAT on different LB medium (see [[Paris/July_11|July 11]] |
Revision as of 10:28, 26 July 2007
Contents |
Preparation of a stock of phage on w121:
David B:
- Filtration of the Cacl2 and MgSO4 stocks
- Launch a culture:
- 15ml LB
- 225µl Cacl2
- 450µl MgSO4
- 90µl DAP
- 150µl W121 from an ON culture
- Let grow until OD=0.2 (~1h)
- Add 100µl P1 (stock 1010/ml)
- Incubate 2h
- Add 200µl Chloroform
- Vortex, centrifuge (15min @ 3000rpm)
- Recover the supernatant + add 200µl Chloroform = Stock keep at 4°C
Titration of bacteriophages P1
Nicolas C.
We measure the strength of former P1 preparation :
- The stock prepared the 3.7.7
- The stock prepared the 8.7.7
- The stock prepared today (12.7.7)
See results.
See protocols for details.
Culture of FtsZ TS
David B
We want to isolate a good clone of the strain FstZ TS
From the plate of FtsZ TS84 121:
isolation of 6 clones of the 121.1 on LBA+tet incubated at 42°C + Culture ON at 30°C
If one clone do not grow at 42, we'll use it to do the transduction.
See result.
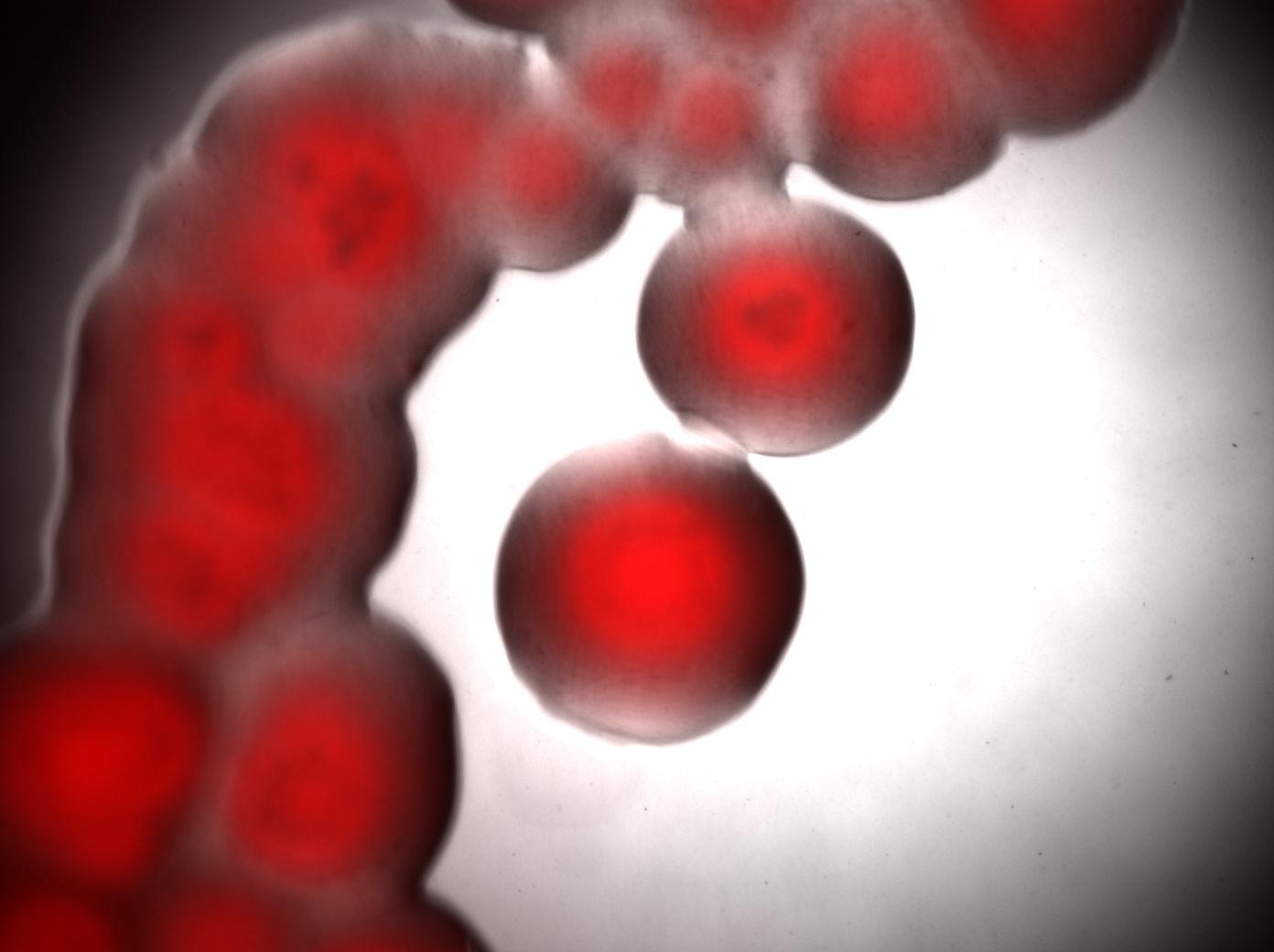
Acinetobacter microscopy
We looked under 5X fluorescence microscopy the fluorescence of colonies of Acinetobacter incubated on LNMM Nile Red for 4-5 days:
Visualisation of colonies stained by Nile Red. It seems that only cells in the middle of colonies (i.e. "old" cells) are producing triglycerides whereas cells on the edges doesn't. Without Nile red, the colonies show no fluorescence.
E.coli pKS::DGAT on LB oletate medium
We incubated E.coli pKS::DGAT on different LB medium (see July 11