Imperial/Dry Lab/Modelling
From 2007.igem.org
(→Model Development for Infector Detector) |
(→Selection of Model Design and Structure) |
||
| Line 16: | Line 16: | ||
==Selection of Model Design and Structure== | ==Selection of Model Design and Structure== | ||
| + | |||
| + | Since the novelty of our solution revolves around the use of cell-free systems as a baterial-free solution in the clinical setting, a simple system is selected. This solution, involves the proposal of two simple constructs, varying with respect to the manner in which LuxR is introduced into the system: | ||
| + | *Construct 1 - represented by [http://partsregistry.org/Part:BBa_T9002| T9002], incorporates constitutive expression of LuxR by pTET. | ||
| + | *Construct 2 - simpler in nature, lacks pTET; LuxR is introduced in purified form here.<br> | ||
| + | |||
| + | <font color = red>~~Here explain briefly why Construct 2 was selected, i.e. we were concerned with the time the system would take to reach steady-state (that is before energy-dependence was considered) - due to almost negligible <math> \delta_{LuxR}</math>, etc </font>. | ||
==References== | ==References== | ||
Revision as of 22:25, 21 October 2007
Contents |
Model Development for Infector Detector
Formulation of the problem
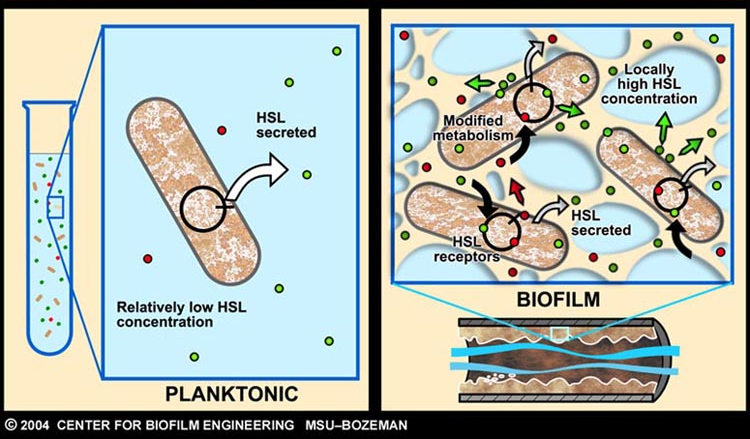
As described earlier, catheter-associated urinary tract infection (CAUTI) in the clinical setting is a prevalent problem with extensive economic impact. The underlying cause of many such infections can be attributed to the formation of biofilm, by aggregating-bacteria on the surface of urinary catheters.
Infector Detector (ID) is a simple biological detector, which serves to expose bacterial biofilm. It functions by exploiting the inherent AHL (Acetyl Homoserine Lactone) production employed by certain types of quorum-sensing bacteria, in the formation of such structures.
Our project attempts to improve where previous methods of biofilm detection have proven ineffective: first and foremost, by focussing on the sensitivity of the system, to markers of biofilm: in this case, low levels of AHL production (which represents the bacterial "chatter" of such aggregating bacteria).
In doing so, a complete investigation of the level of sensitivity to AHL concentration needs to be performed - in other words, what is the minimal AHL concentration for appreciable expression of a chosen reporter protein. Furthermore, establish a functional range for possible AHL detection. How does increased AHL concentration impact on the maximal output of reporter protein?
Finally, how can the system performance be tailored, by exploiting possible state variables (e.g. varying initial LuxR concentration and/or concentration of pLux promoters).
The system performance here revolves most importantly around AHL sensitivity; however, the effect on the maximal output of fluorescent reporter protein and response time is, likewise, of great importance.
Selection of Model Design and Structure
Since the novelty of our solution revolves around the use of cell-free systems as a baterial-free solution in the clinical setting, a simple system is selected. This solution, involves the proposal of two simple constructs, varying with respect to the manner in which LuxR is introduced into the system:
- Construct 1 - represented by [http://partsregistry.org/Part:BBa_T9002| T9002], incorporates constitutive expression of LuxR by pTET.
- Construct 2 - simpler in nature, lacks pTET; LuxR is introduced in purified form here.
~~Here explain briefly why Construct 2 was selected, i.e. we were concerned with the time the system would take to reach steady-state (that is before energy-dependence was considered) - due to almost negligible <math> \delta_{LuxR}</math>, etc .